EMERGENCY CONTRACEPTIVE- levonorgestrel tablet
Rugby Laboratories, Inc.
----------
EContra One-Step®
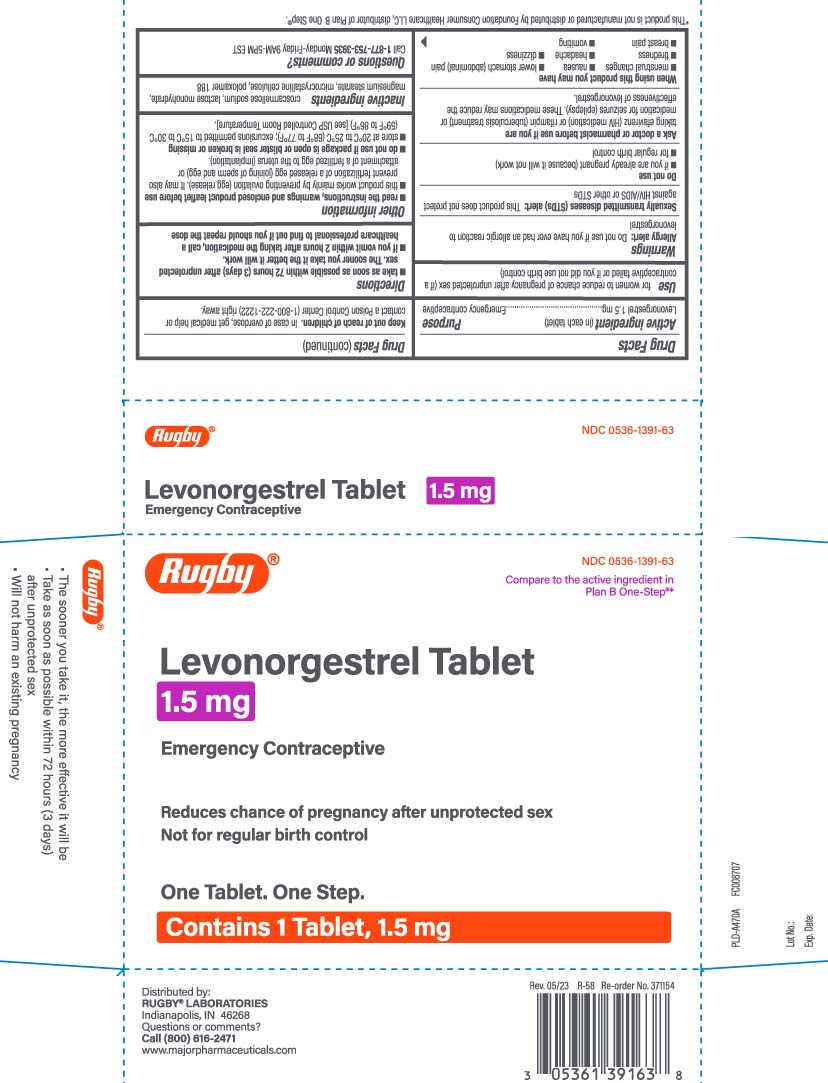
(Levonorgestrel) Tablet 1.5 mg
Use
for women to reduce chance of pregnancy after unprotected sex (if a contraceptive failed or if you did not use birth control)
Warnings
Allergy alert: Do not use if you have ever had an allergic reaction to levonorgestrel
Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs
Keep out of reach children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- take as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it the better it will work.
-
if you vomit within 2 hours after taking the medication, call a healthcare professional to find out if you should repeat the dose
Other information
- read the instructions, warnings and enclosed product leaflet before use
- this product works mainly by preventing ovulation (egg release). It may also prevent fertilization of a released egg (joining of sperm and egg) or attachmentof a fertilized egg to the uterus (implantation).
- do not use if package is open or blister seal is broken or missing
- store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
Inactive ingredients
croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer 188
Principal display panel
Compare to the active ingredient in Plan B One-Step**
Levonorgestrel Tablet 1.5 mg
Emergency Contraceptive
Reduces chances of pregnancy after unprotected sex
Not for regular birth control
One Tablet. One Step
- The sooner you take it, the more effective it will be
- Take as soon as possible within 72 hours (3 days) after unprotected sex
- Will not harm an existing pregnancy
Tablet
*This product is not manufactured or distributed by Foundation Consumer Healthcare LLC, distributor of Plan B One Step®.
Distributed by:
RUGBY LABORATORIES
Indianapolis, IN 46268
| EMERGENCY CONTRACEPTIVE
levonorgestrel tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rugby Laboratories, Inc. (079246066) |