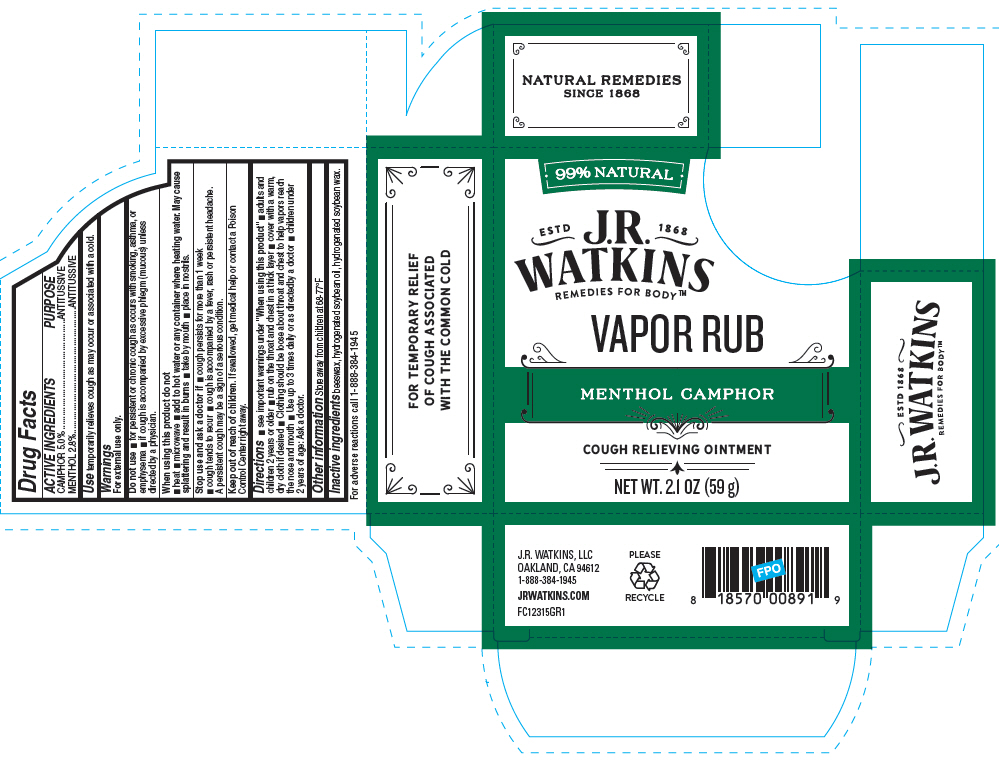
MENTHOL CAMPHOR VAPOR RUB- camphor (natural) and menthol, unspecified form ointment
J.R. Watkins, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Menthol Camphor Vapor Rub
Warnings
For external use only.
Do not use
- for persistent or chronic cough as occurs with smoking, asthma, or emphysema
- if cough is accompanied by excessive phlegm (mucous) unless directed by a physician.
When using this product do not
- heat
- microwave
- add to hot water or any container where heating water. May cause splattering and result in burns
- take by mouth
- place in nostrils.
Directions
- see important warnings under "When using this product"
- adults and children 2 years or older
- rub on the throat and chest in a thick layer
- cover with a warm, dry cloth if desired
- Clothing should be loose about throat and chest to help vapors reach the nose and mouth
- Use up to 3 times daily or as directedby a doctor
- children under 2 years of age: Ask a doctor.
| MENTHOL CAMPHOR VAPOR RUB
camphor (natural) and menthol, unspecified form ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - J.R. Watkins, LLC. (081071049) |
Revised: 8/2023
Document Id: 5a00447f-548c-4da2-b5cc-aba718dd7675
Set id: 48e541dc-f35c-4836-93bb-475201ba7b75
Version: 3
Effective Time: 20230815
J.R. Watkins, LLC.