PROCURE HYDROCORTISONE PLUS CALENDULA- hydrocortisone cream
Profoot, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ProCure Hydrocortisone Cream + Calendula
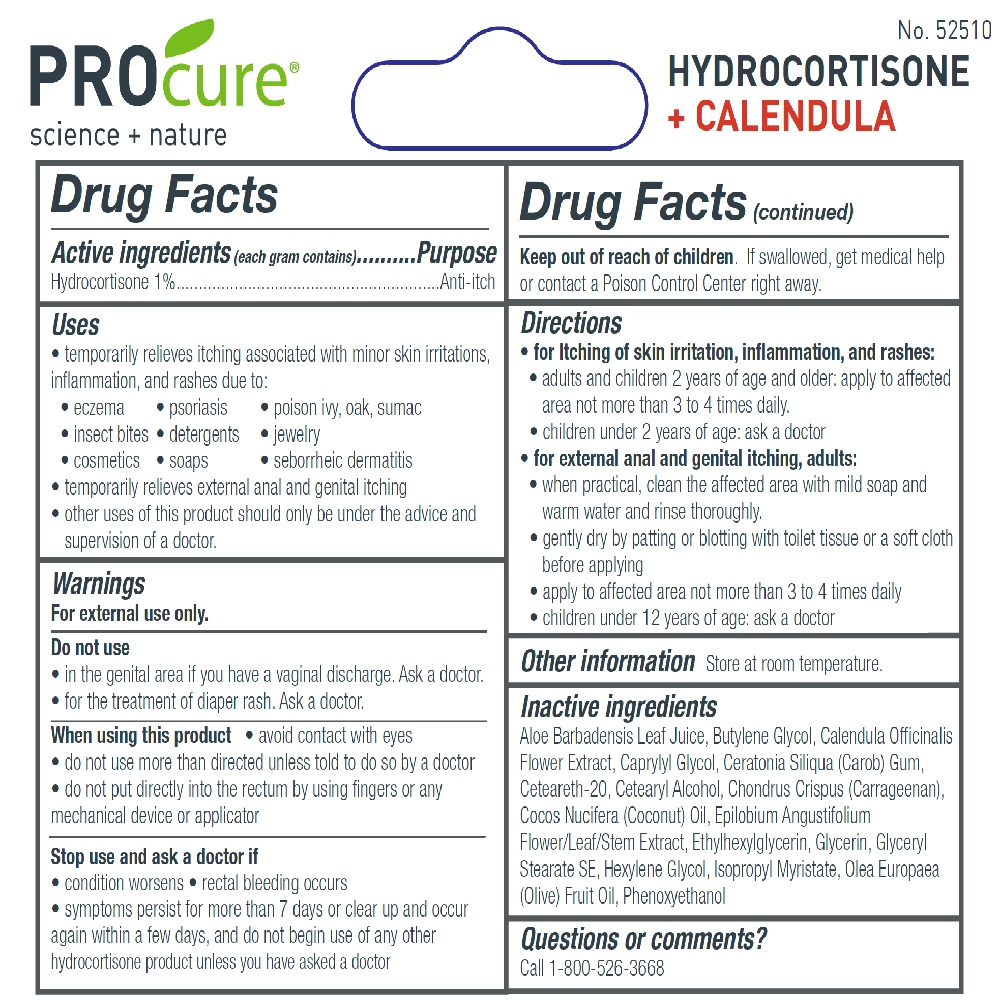
Uses
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
• eczema • psoriasis • poison ivy, oak, sumac • insect bites • detergents • jewelry • cosmetics • soaps • seborrheic dermatitis
- temporarily relieves external anal and genital itching
- other uses of this product should only be under the advice and supervision of a doctor.
Do not use
- in the genital area if you have a vaginal discharge. Ask a doctor.
- for the treatment of diaper rash. Ask a doctor.
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
- do not put directly into the rectum by using fingers or any mechanical device or applicator
Stop use and ask a doctor if
- condition worsens
- rectal bleeding occurs
- symptoms persist for more than 7 days or clear up and occur again within a few days, and do not begin use of any other hydrocortisone product unless you have asked a doctor
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- for Itching of skin irritation, inflammation, and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 2 years of age: ask a doctor
- for external anal and genital itching, adults:
- when practical, clean the affected area with mild soap and warm water and rinse thoroughly.
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- apply to affected area not more than 3 to 4 times daily • children under 12 years of age: ask a doctor
Inactive ingredients
Aloe Barbadensis Leaf Juice, Butylene Glycol, Calendula Officinalis Flower Extract, Caprylyl Glycol, Ceratonia Siliqua (Carob) Gum, Ceteareth-20, Cetearyl Alcohol, Chondrus Crispus (Carrageenan), Cocos Nucifera (Coconut) Oil, Epilobium Angustifolium Flower/Leaf/Stem Extract, Ethylhexylglycerin, Glycerin, Glyceryl Stearate SE, Hexylene Glycol, Isopropyl Myristate, Olea Europaea (Olive) Fruit Oil, Phenoxyethanol
| PROCURE
HYDROCORTISONE PLUS CALENDULA
hydrocortisone cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Profoot, Inc. (107570900) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Concept Laboratories, Inc. | 962282612 | manufacture(29784-171) | |