Label: MATCH PERFECTION SPF 18 SOFT BEIGE 240- octinoxate titanium dioxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 76485-1032-1 - Packager: Rimmel Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 18, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
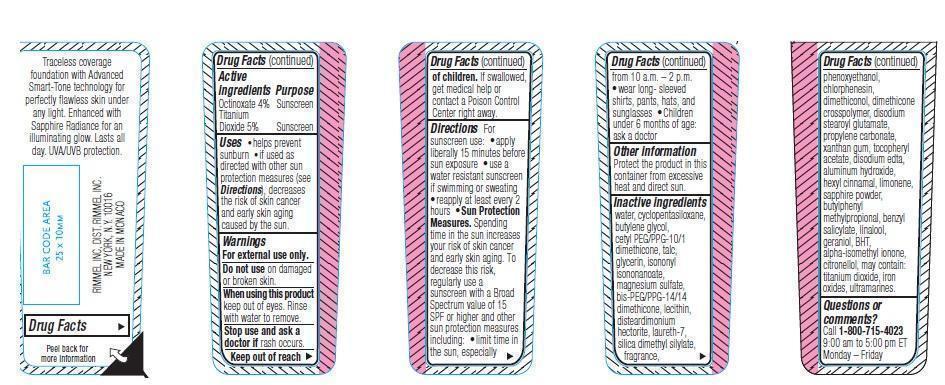
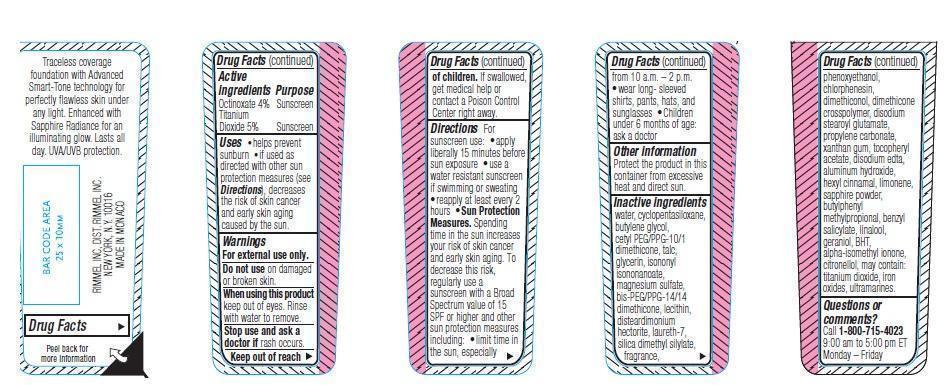
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions. For sunscreen use: apply liberally 15 minutes before sun exposure. Use a water resistant sunscreen if swimming or sweating. Reapply at least every 2 hours.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of 15 SPF or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
water, cyclopentasiloxane, butylene blycol, cetyl PEG/PPG-10/1 dimethicone, talc, glycerin, isononyl isononanoate, magnesium sulfate, bis-PEG/PPG-14/14 dimethicone, lecithin, disteardimonium hectorite, laureth-7, silica dimethyl silylate, fragrance, phenoxyethanol, chlorphenesin, dimethiconol, dimethicone, crosspolymer, disodium stearoyl glutamate, propylene carbonate, xanthan gum, tocopheryl acetate, disodium edta, aluminum hydroxide, hexyl cinnamal, limonene, sapphire powder, butylphenyl methylpropional, benzyl salicylate, linalool, geraniol, BHT, alpha-isomethyl ionone, citronellol, may contain: titanium dioxide, iron oxides, ultramarines.
- Front Labels
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MATCH PERFECTION SPF 18 SOFT BEIGE 240
octinoxate titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76485-1032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.2 mL in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.5 mL in 30 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) TALC (UNII: 7SEV7J4R1U) GLYCERIN (UNII: PDC6A3C0OX) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) MAGNESIUM SULFATE (UNII: DE08037SAB) LAURETH-7 (UNII: Z95S6G8201) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) PROPYLENE CARBONATE (UNII: 8D08K3S51E) XANTHAN GUM (UNII: TTV12P4NEE) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) BENZYL SALICYLATE (UNII: WAO5MNK9TU) LINALOOL, (+/-)- (UNII: D81QY6I88E) GERANIOL (UNII: L837108USY) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76485-1032-1 30 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/15/2012 Labeler - Rimmel Inc. (949791073) Establishment Name Address ID/FEI Business Operations Lancaster S.A.M. 401011325 manufacture(76485-1032)