Label: MECLIZINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 12634-424-00, 12634-424-01, 12634-424-09, 12634-424-12, view more12634-424-18, 12634-424-40, 12634-424-42, 12634-424-45, 12634-424-50, 12634-424-52, 12634-424-54, 12634-424-57, 12634-424-59, 12634-424-60, 12634-424-61, 12634-424-63, 12634-424-66, 12634-424-67, 12634-424-69, 12634-424-71, 12634-424-74, 12634-424-78, 12634-424-79, 12634-424-80, 12634-424-81, 12634-424-82, 12634-424-84, 12634-424-85, 12634-424-90, 12634-424-91, 12634-424-92, 12634-424-93, 12634-424-94, 12634-424-95, 12634-424-96, 12634-424-97, 12634-424-98, 12634-424-99 - Packager: Apotheca Inc.
- This is a repackaged label.
- Source NDC Code(s): 42806-014
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 25, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
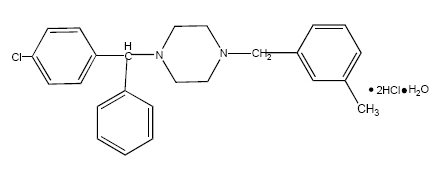
Chemically, meclizine hydrochloride is 1-( p-chloro-α-phenylbenzyl)-4-( m-methylbenzyl) piperazine dihydrochloride monohydrate.
C 25H 27CIN 2·2HCl·H 20 M.W. 481.89
Meclizine hydrochloride tablets, USP are available in two different strengths, 12.5 mg and 25 mg. Inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium and magnesium stearate. The 12.5 mg tablet also contains FD&C Blue #1 Aluminum Lake.
-
CLINICAL PHARMACOLOGY
Meclizine hydrochloride is an antihistamine that shows marked protective activity against nebulized histamine and lethal doses of intravenously injected histamine in guinea pigs. It has a marked effect in blocking the vasodepressor response to histamine, but only a slight blocking action against acetylcholine. Its activity is relatively weak in inhibiting the spasmogenic action of histamine on isolated guinea pig ileum.
Pharmacokinetics
The available pharmacokinetic information for meclizine following oral administration has been summarized from published literature.
Absorption
Meclizine is absorbed after oral administration with maximum plasma concentrations reaching at a median T max value of 3 hours post-dose (range: 1.5 to 6 hours) for the tablet dosage form.
Metabolism
The metabolic fate of meclizine in humans is unknown. In an in vitro metabolic study using human hepatic microsome and recombinant CYP enzyme, CYP 2D6 was found to be the dominant enzyme for metabolism of meclizine.
The genetic polymorphism of CYP2D6 that results in extensive-, poor-, intermediate- and ultrarapid metabolizer phenotypes could contribute to large inter-individual variability in meclizine exposure.
-
INDICATIONS
Based on a review of this drug by the National Academy of Sciences - National Research Council and/or other information, FDA has classified the indications as follows:
Effective: Management of nausea and vomiting, and dizziness associated with motion sickness.
Final classification of the less than effective indications requires further investigation.
- CONTRAINDICATIONS
-
WARNINGS
Since drowsiness may, on occasion, occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking this drug.
Due to its potential anticholinergic action, this drug should be used with caution in patients with asthma, glaucoma, or enlargement of the prostate gland.
Usage in Children
Clinical studies establishing safety and effectiveness in children have not been done; therefore, usage is not recommended in children under 12 years of age.
Usage in Pregnancy
Pregnancy Category B. Reproduction studies in rats have shown cleft palates at 25-50 times the human dose. Epidemiological studies in pregnant women, however, do not indicate that meclizine increases the risk of abnormalities when administered during pregnancy. Despite the animal findings, it would appear that the possibility of fetal harm is remote. Nevertheless, meclizine, or any other medication, should be used during pregnancy only if clearly necessary.
-
PRECAUTIONS
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when meclizine is administered to a nursing woman.
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of meclizine has not been evaluated. As meclizine undergoes metabolism, hepatic impairment may result in increased systemic exposure of the drug. Treatment with meclizine should be administered with caution in patients with hepatic impairment.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of meclizine has not been evaluated. Due to a potential drug/metabolite accumulation, meclizine should be administered with caution in patients with renal impairment and in the elderly as renal function generally declines with age.
Drug Interactions
There may be increased CNS depression when meclizine is administered concurrently with other CNS depressants, including alcohol, tranquilizers, and sedatives. (see WARNINGS)
Based on in-vitro evaluation, meclizine is metabolized by CYP2D6. Therefore there is a possibility for a drug interaction between meclizine and CYP2D6 inhibitors.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
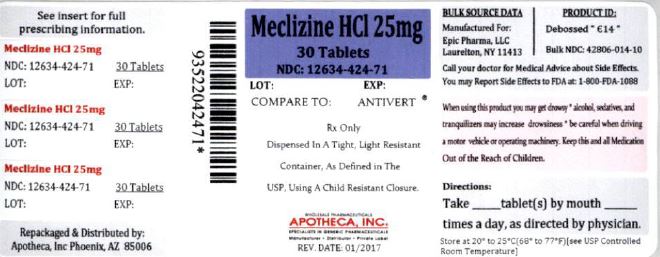
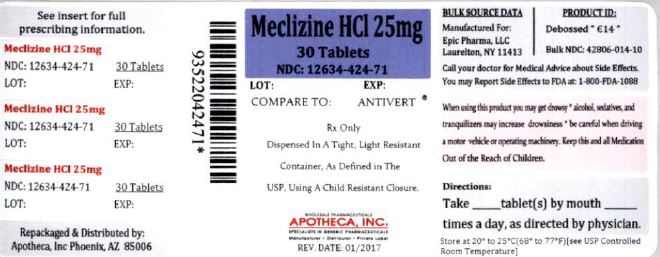
25 mg, white, modified oval-shaped tablets, de-bossed “ Є14” on one side and plain on the other side. They are supplied as follows:
NDC 12634-424-00 Bottles of 10
NDC 12634-424-01 Bottles of 100
NDC 12634-424-09 Bottles of 35
NDC 12634-424-12 Bottles of 120
NDC 12634-424-18 Bottles of 180
NDC 12634-424-40 Bottles of 40
NDC 12634-424-42 Bottles of 42
NDC 12634-424-45 Bottles of 45
NDC 12634-424-50 Bottles of 50
NDC 12634-424-52 Blister Pack of 12
NDC 12634-424-54 Blister Pack of 14
NDC 12634-424-57 Blister Pack of 20
NDC 12634-424-59 Blister Pack of 30
NDC 12634-424-60 Bottles of 60
NDC 12634-424-61 Blister Pack of 10
NDC 12634-424-63 Blister Pack of 3
NDC 12634-424-66 Blister Pack of 6
NDC 12634-424-67 Blister Pack of 7
NDC 12634-424-69 Blister Pack of 9
NDC 12634-424-71 Bottles of 30
NDC 12634-424-74 Bottles of 24
NDC 12634-424-78 Bottles of 28
NDC 12634-424-79 Bottles of 25
NDC 12634-424-80 Bottles of 20
NDC 12634-424-81 Bottles of 21
NDC 12634-424-82 Bottles of 12
NDC 12634-424-84 Bottles of 14
NDC 12634-424-85 Bottles of 15
NDC 12634-424-90 Bottles of 90
NDC 12634-424-91 Blister Pack of 1
NDC 12634-424-92 Bottles of 2
NDC 12634-424-93 Bottles of 3
NDC 12634-424-94 Bottles of 4
NDC 12634-424-95 Bottles of 5
NDC 12634-424-96 Bottles of 6
NDC 12634-424-97 Bottles of 7
NDC 12634-424-98 Bottles of 8
NDC 12634-424-99 Bottles of 9
Store at 20º to 25ºC (68 to 77ºF) [See USP Controlled Room Temperature]. Dispense contents in a tight, light-resistant container as defined in the USP, with a child-resistant closure, as required.
Manufactured by:
Epic Pharma, LLC
Laurelton, NY 11413
Manufactured in USA
Revised January 2016
MF012REV01/16
OE1035
Repackaged & Distributed by:
Apotheca Inc.
Phoenix, AZ 85006
- PRINCIPAL DISPLAY PANEL – 25 mg, 30 Tablets
-
INGREDIENTS AND APPEARANCE
MECLIZINE HYDROCHLORIDE
meclizine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12634-424(NDC:42806-014) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape OVAL Size 13mm Flavor Imprint Code E14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12634-424-00 10 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 2 NDC:12634-424-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 3 NDC:12634-424-09 35 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 4 NDC:12634-424-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 5 NDC:12634-424-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 6 NDC:12634-424-40 40 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 7 NDC:12634-424-42 42 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 8 NDC:12634-424-45 45 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 9 NDC:12634-424-50 50 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 10 NDC:12634-424-52 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 11 NDC:12634-424-54 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 12 NDC:12634-424-57 20 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 13 NDC:12634-424-59 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 14 NDC:12634-424-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 15 NDC:12634-424-61 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 16 NDC:12634-424-63 3 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 17 NDC:12634-424-66 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 18 NDC:12634-424-67 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 19 NDC:12634-424-69 9 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 20 NDC:12634-424-71 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 21 NDC:12634-424-74 24 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 22 NDC:12634-424-78 28 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 23 NDC:12634-424-79 25 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 24 NDC:12634-424-80 20 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 25 NDC:12634-424-81 21 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 26 NDC:12634-424-82 12 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 27 NDC:12634-424-84 14 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 28 NDC:12634-424-85 15 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 29 NDC:12634-424-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 30 NDC:12634-424-91 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/30/2012 31 NDC:12634-424-92 2 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 32 NDC:12634-424-93 3 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 33 NDC:12634-424-94 4 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 34 NDC:12634-424-95 5 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 35 NDC:12634-424-96 6 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 36 NDC:12634-424-97 7 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 37 NDC:12634-424-98 8 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 38 NDC:12634-424-99 9 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200294 04/30/2012 Labeler - Apotheca Inc. (051457844) Establishment Name Address ID/FEI Business Operations Apotheca Inc. 051457844 relabel(12634-424) , repack(12634-424)