Label: XARACOLL- bupivacaine hcl collagen-matrix implant
-
Contains inactivated NDC Code(s)
NDC Code(s): 51715-100-04, 51715-100-10 - Packager: INNOCOLL PHARMACEUTICALS
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 8, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XARACOLL® safely and effectively. See full prescribing information for XARACOLL.
XARACOLL® (bupivacaine hydrochloride) implant
Initial U.S. Approval: 1972INDICATIONS AND USAGE
XARACOLL contains an amide local anesthetic and is indicated in adults for placement into the surgical site to produce postsurgical analgesia for up to 24 hours following open inguinal hernia repair (1).
Limitations of Use
Safety and effectiveness have not been established in other surgical procedures, including orthopedic and boney procedures (5.5).DOSAGE AND ADMINISTRATION
- XARACOLL is intended for single-dose administration. The recommended dose is 300 mg bupivacaine HCl (three XARACOLL implants, each containing 100 mg bupivacaine HCl) (2.2).
- Each XARACOLL implant should be cut in half using aseptic technique prior to placing the dry implants into the surgical site. Place three halves below the site of mesh placement and three halves just below the skin closure. (2.3).
DOSAGE FORMS AND STRENGTHS
100 mg per implant (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Dose-Related Toxicity: Monitor cardiovascular and respiratory vital signs and patient’s state of consciousness after placement of XARACOLL. (5.1)
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. See full prescribing information for more detail on managing these risks (5.2).
ADVERSE REACTIONS
Most common adverse reactions in clinical trials (incidence ≥2% and higher than placebo) included incision site swelling, dysgeusia, headache, tremor, vision blurred, seroma, scrotal swelling, pyrexia, hypoesthesia oral, and post procedural discharge. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Innocoll at 1-833-606-1421 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Local Anesthetics: The toxic effects of local anesthetics are additive. Avoid additional local anesthetic administration within 96 hours following XARACOLL implantation. If additional local anesthetic administration with XARACOLL cannot be avoided, monitor patients for neurologic and cardiovascular effects related to local anesthetic systemic toxicity (7.1).
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
2.2 Recommended Dose
2.3 Placement Instructions
2.4 Compatibility Considerations
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Dose-related Toxicity
5.2 Methemoglobinemia
5.3 Risk of Toxicity in Patients with Hepatic Impairment
5.4 Risk of Use in Patients with Impaired Cardiovascular Function
5.5 Risk of Delayed Bone Healing with Unapproved Use
6. ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7. DRUG INTERACTIONS
7.1 Local Anesthetics
7.2 Drugs Associated with Methemoglobinemia
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10. OVERDOSAGE
11. DESCRIPTION
11.1 Active Ingredient
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1. INDICATIONS AND USAGE
XARACOLL is indicated in adults for placement into the surgical site to produce postsurgical analgesia for up to 24 hours following open inguinal hernia repair.
Limitations of Use
Safety and effectiveness have not been established in other surgical procedures, including orthopedic and boney procedures [see Warnings and Precautions (5.5)].
-
2. DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
- XARACOLL is to be administered by or under the supervision of experienced clinicians who are well versed in the diagnosis and management of dose-related toxicity and other acute emergencies which might arise from bupivacaine exposure.
- The toxic effects of local anesthetics are additive. Avoid additional local anesthetic administration within 96 hours following XARACOLL implantation. If additional local anesthetic administration with XARACOLL cannot be avoided based on clinical need, monitor patients for neurologic and cardiovascular effects related to local anesthetic systemic toxicity [see Warnings and Precautions (5.1), Drug Interactions (7), Overdosage (10)].
- Use XARACOLL only if the following are immediately available: oxygen, other resuscitative drugs, cardiopulmonary resuscitative equipment, and the personnel resources needed for proper management of toxic reactions and related emergencies [see Warnings and Precautions (5.1), Adverse Reactions (6), Overdosage (10)].
- XARACOLL is supplied as a sterile product that should be handled using aseptic technique. XARACOLL is designed as a ready-to-use product and requires no preparation other than cutting the individual implants as needed to accommodate the surgical space.
- Each single-dose package (pouch) of XARACOLL contains three implants comprising the total dose of 300 mg bupivacaine HCl. Inspect the outer pouch and inner blister packaging prior to use. Do not use XARACOLL if the packaging has been compromised.
- XARACOLL is white to off-white in color, has uniform thickness, and is approximately 5 cm × 5 cm × 0.5 cm in size. Do not use XARACOLL if it appears discolored, contains foreign particulates, or is collapsed, compressed, or misshapen.
- Avoid excessive handling and compression of XARACOLL.
- Avoid contact of XARACOLL with liquids prior to placement. Place the XARACOLL implant into the surgical site dry. Pre-moistening may result in premature release of bupivacaine from XARACOLL.
2.2 Recommended Dose
- XARACOLL is intended for single-dose administration. The recommended dose of XARACOLL is 300 mg (3 x 100 mg implants).
- Doses of XARACOLL above 300 mg (3 x 100 mg implants) per patient have not been studied in clinical trials.
2.3 Placement Instructions
- Both the outer pouch and the inner blister packaging containing the individual implants are designed to be peeled open. Aseptically peel open the outer pouch, then remove and aseptically peel open the three inner blister packages containing XARACOLL. To avoid cutting the implants prior to placement, do not open the blister packaging using scissors or a scalpel.
- Carefully remove XARACOLL from the inner blister packages and inspect each implant prior to use.
- Using aseptic technique, cut each XARACOLL implant in half prior to placement into the surgical site. Place three halves below the site of mesh placement and three halves just below the skin closure.
- XARACOLL may become difficult to move once placed in the surgical site and moistened. Use care when moving XARACOLL after placement.
2.4 Compatibility Considerations
Administration of additional local anesthetics, including bupivacaine HCl, into the surgical site with XARACOLL has not been studied.
Studies conducted with XARACOLL demonstrated that commonly used surgical materials (nonabsorbable surgical suture, delayed absorbable surgical suture, and surgical mesh) are not affected by the presence of XARACOLL.
When a topical antiseptic such as povidone iodine (e.g., Betadine®) is applied, allow the surgical site to dry before XARACOLL is administered.
- 3. DOSAGE FORMS AND STRENGTHS
-
4. CONTRAINDICATIONS
XARACOLL is contraindicated in:
- patients with a known hypersensitivity to any local anesthetic agent of the amide-type or to any of the other components of XARACOLL.
- patients undergoing obstetrical paracervical block anesthesia. The use of bupivacaine in this technique has resulted in fetal bradycardia and death.
-
5. WARNINGS AND PRECAUTIONS
5.1 Dose-related Toxicity
The safety and effectiveness of local anesthetics depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. The toxic effects of local anesthetics are additive. Avoid additional local anesthetic administration within 96 hours following XARACOLL implantation. If additional local anesthetic administration with XARACOLL cannot be avoided based on clinical need, monitor patients for neurologic and cardiovascular effects related to local anesthetic systemic toxicity. Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be performed after administration of XARACOLL.
Possible early warning signs of central nervous system (CNS) toxicity are restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, CNS depression, or drowsiness. Delay in proper management of dose-related toxicity, underventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and, possibly, death. Consider surgical removal of XARACOLL depending on the clinical situation.
5.2 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious CNS and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Consider removal of XARACOLL and discontinue any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.3 Risk of Toxicity in Patients with Hepatic Impairment
Because amide local anesthetics such as bupivacaine are metabolized by the liver, consider increased monitoring for bupivacaine systemic toxicity in patients with moderate to severe hepatic impairment who are treated with XARACOLL [see Use in Specific Populations (8.6)].
5.4 Risk of Use in Patients with Impaired Cardiovascular Function
Patients with impaired cardiovascular function (e.g., hypotension, heart block) may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by XARACOLL. Monitor patients closely for blood pressure, heart rate, and ECG changes.
5.5 Risk of Delayed Bone Healing with Unapproved Use
The safety and effectiveness of XARACOLL in surgical procedures other than open inguinal hernia repair have not been established, and XARACOLL is not approved for use in these other surgical procedures (e.g., orthopedic procedures). A study evaluating the effects of bupivacaine HCl implant in rats following an osteotomy procedure demonstrated inhibition of bone healing [see Nonclinical Toxicology (13.2)].
-
6. ADVERSE REACTIONS
The following clinically significant adverse reactions have been reported and described in the Warnings section in the labeling:
- Dose-Related Toxicity [see Warnings and Precautions (5.1)]
- Methemoglobinemia [see Warnings and Precautions (5.2)]
6.1 Clinical Trial Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The safety of XARACOLL was evaluated in 11 clinical studies, including two Phase 3 double-blind, placebo implant-controlled studies in patients undergoing open, unilateral inguinal hernia repair. Overall, 612 patients were treated with a single dose of XARACOLL, total dose ranged from 100 mg to 300 mg bupivacaine HCl. Patients treated with XARACOLL ranged in age from 18 to 85 years (median age 51 years), with 88% male, 88% White, 9% African-American, and 3% all other races.
Across the XARACOLL drug development program, which included evaluations in various surgery models, there was one patient death reported in the placebo implant treatment group, and 16 patients who experienced one or more serious adverse events; 11 patients in the XARACOLL treatment group and 5 patients in the placebo implant or comparator treatment groups. Serious adverse reactions reported in the XARACOLL treatment group included wound infection and seroma. There was a single patient who experienced signs and symptoms consistent with local anesthetic systemic toxicity (LAST) approximately four hours after administration of an early formulation of the bupivacaine collagen implant 150 mg during bladder sling surgery. LAST treatment included administration of lipid emulsion and surgical removal of the XARACOLL implants.
The most common adverse reactions (incidence greater than or equal to 2% and higher than placebo implant) following XARACOLL administration were dysgeusia, headache, scrotal swelling, tremor, pyrexia, vision blurred, and seroma. Common incision site adverse reactions (incidence greater than or equal to 2% and higher in either the XARACOLL or placebo implant groups compared to non-implant comparator treatment groups) were swelling pain, other complication, post-procedural discharge, erythema, dehiscence, and inflammation.
Adverse Reactions Reported in Phase 3 Placebo-controlled Trials
There were 619 patients who underwent open inguinal hernia repair in the two Phase 3 studies. Patients received general anesthesia intra-operatively, and a standard acetaminophen regimen, in addition to intravenous and oral morphine as needed, post-operatively. The most common adverse reactions (incidence greater than or equal to 2% and higher than placebo implants) following XARACOLL administration were incision site swelling, dysgeusia, headache, tremor, vision blurred, seroma, scrotal swelling, pyrexia, hypoesthesia oral, and post procedural discharge shown in Table 1.
Table 1: Adverse Reactions (ARs) with Incidence Greater Than or Equal to 2% and Greater Than Placebo Reported in the Phase 3 Placebo-controlled Studies - *
- Placebo consisted of three collagen implants.
XARACOLL 300 mg
N=411
n (%)Placebo*
N=208
n (%)Subjects Reporting Treatment Emergent Adverse Events 256 (62.3%) 143 (68.8%) Injury, Poisoning and Procedural Complications Incision Site Swelling 60 (14.6%) 30 (14.4%) Post Procedural Discharge 20 (4.9%) 10 (4.8%) Seroma 12 (2.9%) 5 (2.4%) Nervous System Disorders Dysgeusia 31 (7.5%) 13 (6.3%) Headache 17 (4.1%) 1 (0.5%) Tremor 15 (3.6%) 6 (2.9%) Gastrointestinal Disorders Hypoaesthesia Oral 9 (2.2%) 4 (1.9%) Reproductive System and Breast Disorders Scrotal Swelling 12 (2.9%) 2 (1.0%) General Disorders and Administration Site Conditions Pyrexia 10 (2.4%) 1 (0.5%) Eye Disorders Vision Blurred 15 (3.6%) 6 (2.9%) 6.2 Postmarketing Experience
The following adverse reactions from voluntary reports have been reported with various formulations of bupivacaine, administered via different routes and for different indications. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions to bupivacaine are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation.
The most commonly encountered acute adverse reactions that demand immediate counter-measures were related to the CNS and the cardiovascular system. These adverse reactions were generally dose-related and due to high plasma levels, which may have resulted from overdosage, rapid absorption from the injection site, diminished tolerance, or from unintentional intravascular injection of the local anesthetic solution.
Nervous System Disorders: Adverse reactions were characterized by excitation and/or depression of the central nervous system and included restlessness, anxiety, dizziness, tinnitus, blurred vision, tremors, convulsions, drowsiness, unconsciousness, respiratory arrest, nausea, vomiting, chills, pupillary constriction.
Neurological effects following routes of administration other than epidural or caudal have included persistent anesthesia, paresthesia, weakness, and paralysis, all with slow, incomplete, or no recovery.
The incidence of adverse neurologic reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient.
Cardiac Disorders: High doses have led to high plasma levels and related depression of the myocardium, decreased cardiac output, heart block, hypotension, bradycardia, ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, and cardiac arrest.
Immune System Disorders: Allergic-type reactions have occurred as a result of sensitivity to bupivacaine or to other formulation ingredients. These reactions were characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and severe hypotension. Cross sensitivity among members of the amide-type local anesthetic group has been reported.
-
7. DRUG INTERACTIONS
7.1 Local Anesthetics
The toxic effects of local anesthetics are additive. Avoid additional local anesthetic administration within 96 hours following XARACOLL implantation. If additional local anesthetic administration with XARACOLL cannot be avoided based on clinical need, monitor patients for neurologic and cardiovascular effects related to local anesthetic systemic toxicity [see Dosage and Administration (2.1), Warnings and Precautions (5.1), Overdosage (10)].
7.2 Drugs Associated with Methemoglobinemia
Patients who are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfasalazine -
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies conducted with XARACOLL in pregnant women to inform a drug-associated risk of adverse development outcomes. In animal studies, embryo-fetal lethality was noted when bupivacaine was administered subcutaneously to pregnant rabbits during organogenesis at clinically relevant doses. Decreased pup survival was observed in a rat pre- and post-natal developmental study (dosing from implantation through weaning) at a dose level comparable to the daily maximum recommended human dose (MRHD). Based on animal data, advise pregnant women of the potential risks to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Labor or Delivery
Local anesthetics rapidly cross the placenta [see Pharmacokinetics (12.3)]. The incidence and degree of toxicity depend upon the procedure performed, the type and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus, and neonate involve alterations of the CNS, peripheral vascular tone, and cardiac function.
Data
Animal Data
Bupivacaine hydrochloride produced developmental toxicity when administered subcutaneously to pregnant rats and rabbits at clinically relevant doses.
Bupivacaine HCl was administered subcutaneously to rats at doses of 4.4, 13.3 and 40 mg/kg and to rabbits at doses of 1.3, 5.8 and 22.2 mg/kg during the period of organogenesis (implantation to closure of the hard palate). No embryo-fetal effects were observed in rats at up to 40 mg/kg, a dose that caused increased maternal lethality. This dose is approximately 1.3 times the daily maximum recommended human dose (MRHD) of 300 mg when calculated on a mg/m2 body surface area (BSA) for a 60 kg woman. An increase in embryo-fetal deaths was observed in rabbits at the high dose (1.4 times the MHRD based on BSA) in the absence of maternal toxicity with the fetal No Observed Adverse Effect Level representing approximately 0.4 times the MRHD on a BSA basis.
In a rat pre- and post-natal development study (dosing from implantation through weaning) conducted at subcutaneous doses of 4.4, 13.3, and 40 mg/kg/day, decreased pup survival was observed at the high dose. The high dose is approximately 1.3 times the daily MRHD on a BSA basis.
8.2 Lactation
Risk Summary
Bupivacaine has been reported to be excreted in human milk suggesting that the nursing infant could be theoretically exposed to a dose of the drug. There is no available information on effects of the drug in the breastfed infant or effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for XARACOLL and any potential adverse effects on the breastfed infant from XARACOLL or from the underlying maternal condition.
8.5 Geriatric Use
Of the total number of patients in the Phase 3 XARACOLL studies (N=411), 60 patients were greater than or equal to 65 years of age and 14 patients were greater than or equal to 75 years of age. No overall differences in efficacy and safety were observed between these patients and younger patients. Clinical experience with XARACOLL has not identified differences in efficacy or safety between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
In clinical studies of bupivacaine, differences in various pharmacokinetic parameters have been observed between elderly and younger patients. Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function. The effects of age (elderly versus younger) on the pharmacokinetics of XARACOLL have not been studied.
8.6 Hepatic Impairment
Amide-type local anesthetics, such as bupivacaine, are metabolized by the liver. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations and potentially local anesthetic systemic toxicity. Consider increased monitoring for local anesthetic systemic toxicity in subjects with moderate to severe hepatic disease [see Warnings and Precautions (5.3), Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Patients with severe renal disease, may be more susceptible to the potential toxicities of the amide-type local anesthetics. Consider increased monitoring for local anesthetic systemic toxicity in subjects with renal disease [see Clinical Pharmacology (12.3)].
-
10. OVERDOSAGE
Clinical Presentation
Acute emergencies from local anesthetics are generally related to high plasma concentrations encountered during therapeutic use of local anesthetics [see Warnings and Precautions (5.1), Adverse Reactions (6)].
If not treated immediately, convulsions with simultaneous hypoxia, hypercarbia, and acidosis plus myocardial depression from the direct effects of bupivacaine may result in cardiac arrhythmias, bradycardia, asystole, ventricular fibrillation, or cardiac arrest. Respiratory abnormalities, including apnea, may occur. If cardiac arrest occurs, successful outcome may require prolonged resuscitative efforts.
Management
The first step in the management of systemic toxic reactions consists of immediate attention to the establishment and maintenance of a patent airway and effective assisted or controlled ventilation with 100% oxygen with a delivery system capable of permitting immediate positive airway pressure by mask. Endotracheal intubation, using drugs and techniques familiar to the clinician, may be indicated after initial administration of oxygen by mask if difficulty is encountered in the maintenance of a patent airway, or if prolonged ventilatory support (assisted or controlled) is indicated.
If necessary, use drugs to manage the convulsions. A bolus intravenous dose of a benzodiazepine will counteract CNS stimulation related to XARACOLL. Immediately after the institution of ventilatory measures, evaluate the adequacy of the circulation. Supportive treatment of circulatory depression may require Advance Cardiac Life Support measures.
Consider surgical removal of XARACOLL depending on the clinical situation.
-
11. DESCRIPTION
XARACOLL contains bupivacaine, an amide local anesthetic, as the active pharmaceutical ingredient. Each XARACOLL collagen implant (5 cm × 5 cm × 0.5 cm) contains 100 mg bupivacaine HCl (equivalent to 88.8 mg bupivacaine) and 75 mg purified Type I collagen.
Each implant is individually packaged in sterile blister packaging. Three implants are packaged in a single-use sterile pouch, for a total of 300 mg bupivacaine HCl (equivalent to 266.4 mg bupivacaine) and 225 mg purified Type I collagen.
The resorbable and biodegradable collagen component of the product serves as an inert delivery system and releases bupivacaine through diffusion from the porous collagen implant, which dissolves over time.
11.1 Active Ingredient
Bupivacaine HCl is a 1-butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide hydrochloride monohydrate, white crystalline powder that is freely soluble in 95% ethanol, soluble in water, and slightly soluble in acetone. The molecular formula of bupivacaine is C18H28N2O and its molecular weight is 288.4. It has the following structural formula:
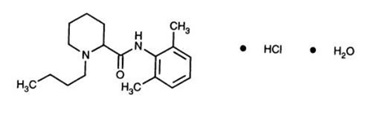
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bupivacaine blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. Clinically, the order of loss of nerve function is (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
12.2 Pharmacodynamics
Systemic absorption of bupivacaine produces effects on the cardiovascular system and CNS. At blood concentrations achieved with normal therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. These cardiovascular changes are more likely to occur after unintended intravascular injection of liquid formulations of bupivacaine.
Following systemic absorption, bupivacaine can produce CNS stimulation, CNS depression, or both. Apparent central stimulation is manifested as restlessness, tremors, and shivering progressing to convulsions, followed by depression and coma progressing ultimately to respiratory arrest. However, bupivacaine has a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited state.
12.3 Pharmacokinetics
Local placement of XARACOLL within the surgical site during open inguinal hernia repair resulted in detectable plasma levels of bupivacaine at the first measured time point (0.5 hours) and throughout the 96-hour observation period [see Warnings and Precautions (5.1)]. Systemic plasma levels of bupivacaine following application of XARACOLL do not correlate with local efficacy.
Absorption
The rate of systemic absorption of bupivacaine is dependent on the total dose administered, the route of administration, and the vascularity of the administration site.
Pharmacokinetic parameters for XARACOLL following placement in the surgical site during hernioplasty are presented in Table 2.
Table 2: Pharmacokinetic Parameters for Bupivacaine After Placement of XARACOLL in the Surgical Site During Open Inguinal Hernia Repair Parameter XARACOLL 300 mg
N=34Cmax (ng/mL)* [minimum, maximum] 663 (264) [274, 1230] Tmax (hours) † [minimum, maximum] 3 [1.5, 24] AUC0-last (h•ng/mL)* 19493 (7564) AUC0-∞ (h•ng/mL)* 20368 (7912) t1/2 (hours)* 19 (6) The highest individual bupivacaine plasma concentration observed in the XARACOLL clinical program was 1230 ng/mL, which occurred 2 hours after placement of the three XARACOLL 100 mg implants (total bupivacaine HCl dose 300 mg) in the surgical site of one patient.
Distribution
After bupivacaine is released from XARACOLL it is absorbed systemically. Local anesthetics including bupivacaine are distributed to some extent to all body tissues, with higher concentrations found in highly perfused organs such as the liver, lungs, heart, and brain.
Local anesthetics including bupivacaine appear to cross the placenta by passive diffusion. The rate and degree of diffusion is governed by (1) the degree of plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility. Fetal/maternal ratios of local anesthetics appear to be inversely related to the degree of plasma protein binding, because only the free, unbound drug is available for placental transfer. Bupivacaine with a high protein binding capacity (95%) has a low fetal/maternal ratio (0.2 to 0.4). The extent of placental transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, non-ionized drugs such as bupivacaine readily enter the fetal blood from the maternal circulation.
Elimination
Metabolism
Amide-type local anesthetics such as bupivacaine are metabolized primarily in the liver via conjugation with glucuronic acid. Pipecoloxylidine is the major metabolite of bupivacaine. The elimination of drug from tissue distribution depends largely upon the availability of binding sites in the circulation to carry it to the liver where it is metabolized.
Excretion
After bupivacaine has been released from XARACOLL and is absorbed systemically, bupivacaine excretion is expected to be the same as for other bupivacaine formulations.
The kidney is the main excretory organ for most local anesthetics and their metabolites. Only 6% of bupivacaine is excreted unchanged in the urine.
Specific Populations
Age
Various pharmacokinetic parameters of the local anesthetics such as bupivacaine can be significantly altered by the age of the patient [see Geriatric Use (8.5)].
Hepatic Impairment
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of hepatic disease. Patients with hepatic disease, especially those with severe hepatic disease, may be more susceptible to the potential toxicities of the amide-type local anesthetics [see Use in Specific Populations (8.6)].
Renal Impairment
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of renal disease, factors affecting urinary pH, and renal blood flow [see Warnings and Precautions (5.1), Use in Specific Populations (8.7), Geriatric Use (8.5)].
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been conducted.
Mutagenesis
Bupivacaine was not mutagenic or clastogenic in a bacterial reverse mutation assay, an in vitro mammalian cell gene mutation test, and an in vivo mammalian erythrocyte micronucleus assay.
Impairment of Fertility
The effect of bupivacaine on fertility has not been determined.
-
14. CLINICAL STUDIES
The efficacy and safety of XARACOLL were evaluated in two randomized, multi-center, double-blind, placebo-controlled Phase 3 trials in patients undergoing open inguinal repair under general anesthesia.
In Study 1, 298 patients were enrolled. The mean age was 53.2 years (range 19 to 86) and patients were predominantly male (96%). In Study 2, 312 patients were enrolled. The mean age was 49.7 years (range 18 to 85) and patients were predominantly male (98%). In each study, three XARACOLL implants, containing 100 mg bupivacaine HCl each, were cut in half. Three halves were placed into the hernia repair site below the site of mesh placement. The muscle/fascial layer was closed and the remaining three halves were placed between the fascia/muscle closure and the skin closure. The placebo consisted of three implants without bupivacaine HCl, similarly prepared and placed. Use of low-dose lidocaine, administered topically or subcutaneously for intravenous catheter placement, or administered intravenously during the induction of general anesthesia prior to surgery and placement of XARACOLL, was reported.
Pain intensity was rated by the patients using a 0 to 10 numerical rating scale at multiple time points up to 72 hours. Immediately postoperatively, patients were allowed parenteral morphine rescue medication as needed. Once tolerating oral intake, patients received a standard acetaminophen regimen (650 mg orally three times daily) and immediate-release oral morphine (15 mg) was available as needed.
The primary outcome measure was the time-weighted sum of pain intensity from Time 0 through 24 hours (SPI24). The secondary endpoints were total use of opioid analgesia from Time 0 through 24 hours (TOpA24), time-weighted sum of pain intensity from Time 0 through 48 hours (SPI48), total use of opioid analgesia from Time 0 through 48 hours (TOpA48), time-weighted sum of pain intensity from Time 0 through 72 hours (SPI72), and total use of opioid analgesia from Time 0 through 72 hours (TOpA72).
In both Study 1 and Study 2, there was a statistically significant treatment effect for XARACOLL compared to placebo in SPI 24 and TOpA24. There was no statistically significant treatment effect for XARACOLL compared to placebo in SPI72 and TOpA72. Table 3 shows the mean sum of pain intensity over the first 24 hours after surgery.
Table 3: Mean Sum of Pain Intensity Over the First 24 Hours After Surgery (Primary Endpoint) Study 1 Study 2 XARACOLL
N=197Placebo1
N=101XARACOLL
N=207Placebo*
N=105SPI24†
Mean (SD)
85.9 (47.2)106.8 (48.2) 88.3 (47.0) 116.2 (44.0) Difference‡
95% CI-20.8
(-32.2, -9.4)-27.8
(-38.6, -17.1)SD=standard deviation; CI=confidence interval;
SPI (sum of pain intensity):The proportion of patients who did not receive opioid rescue analgesia through 72 hours in the XARACOLL and placebo treatment groups was 36% and 22%, respectively, in Study 1, and 28% and 12%, respectively, in Study 2. The median time to first opioid rescue analgesia in the XARACOLL and placebo treatment groups was 11 hours and 1 hour, respectively, in Study 1, and 6 hours and 1 hour, respectively in Study 2.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
XARACOLL (bupivacaine HCl) implant is supplied as three white to off-white sterile surgical implants (approximately 5 cm × 5 cm × 0.5 cm), each containing 100 mg bupivacaine HCl in individually sealed blister packages. A tray of three blister packages in a sterile pouch is provided in one carton. XARACOLL is available as:
- Four single-use cartons, each containing one pouch containing 3 x 100 mg implants (NDC 51715-100-04)
- Ten single-use cartons, each containing one pouch containing 3 x 100 mg implants (NDC 51715-100-10)
Storage
XARACOLL should be stored at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
Handling
Prior to surgical placement:
- Do not use if pouch or blister packaging has been compromised
- Avoid excessive handling
- Keep away from moisture
- Maintain sterility
-
17. PATIENT COUNSELING INFORMATION
Allergic-Type Reactions
Assess if the patient has had allergic-type reactions to amide-type local anesthetics or to other formulation ingredients [see Contraindications (4), Adverse Reactions (6)].
Methemoglobinemia
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Innocoll Pharmaceuticals Limited
Athlone, Ireland N37 VW42USA Patent Number: RE47,826 E
Sep 2020; Version 1.6
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
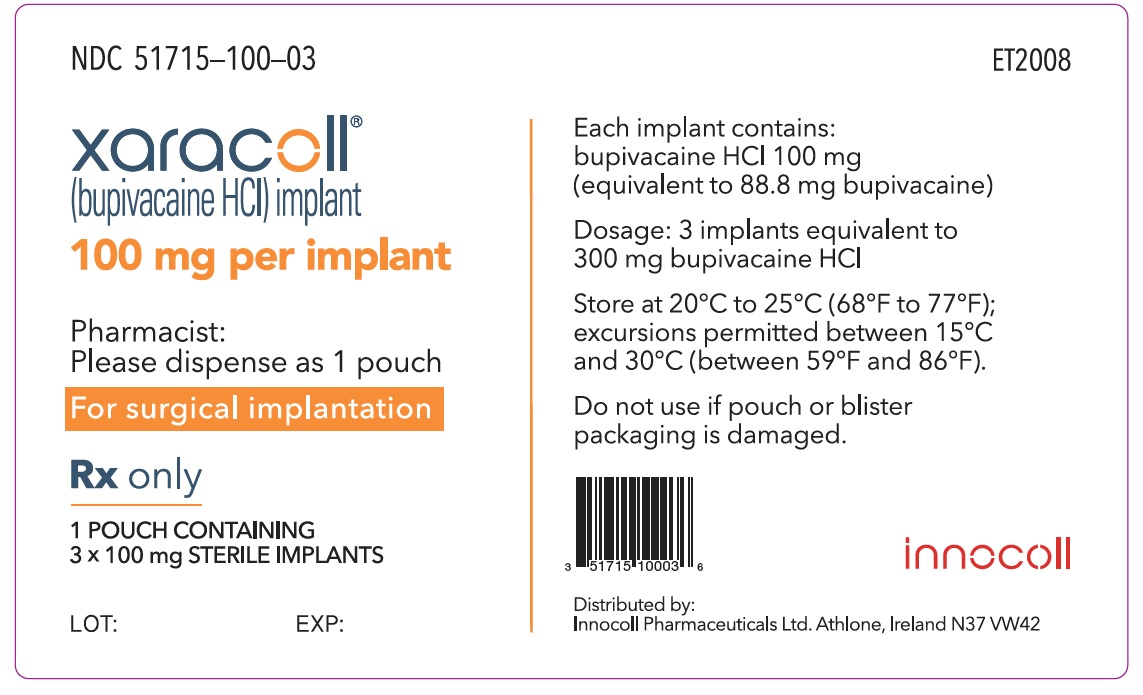
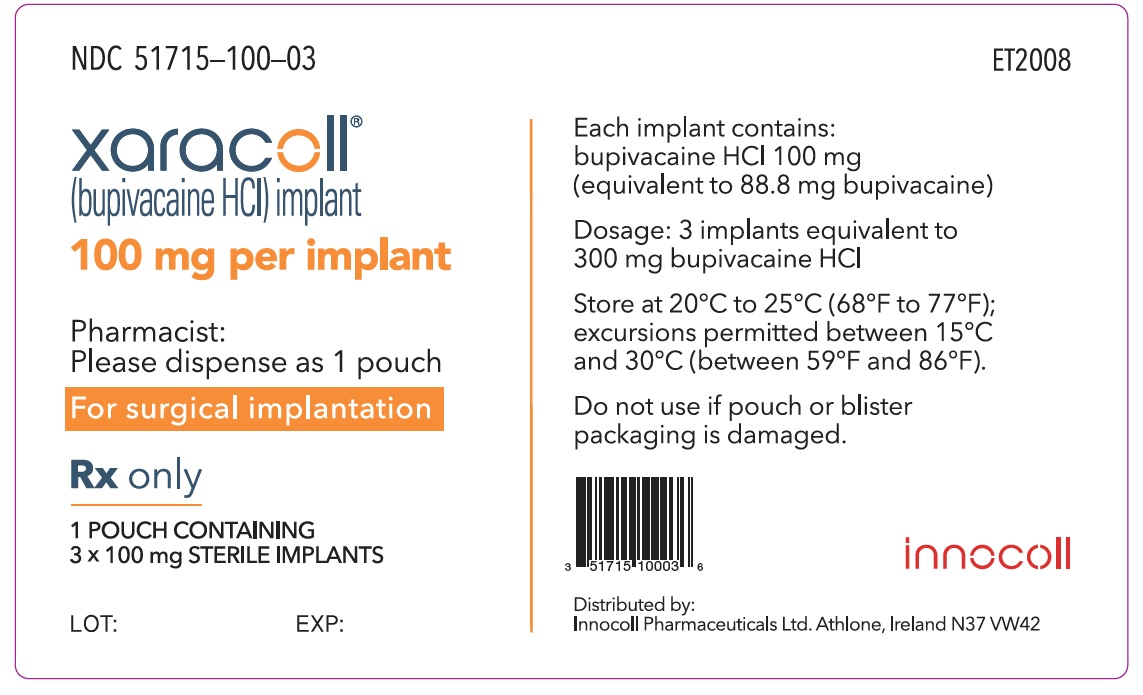
NDC 51715-100-03
xaracoll®
(bupivacaine HCl) implant
100 mg per implant
Rx only
1 POUCH CONTAINING 3 x 100 mg STERILE IMPLANTS
Each implant contains: bupivacaine HCl 100 mg (equivalent to 88.8 mg bupivacaine)
Dosage: 3 implants equivalent to 300 mg of bupivacaine HCl
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F).
Do not use if pouch or blister packaging is damaged.
Pharmacist: Please dispense as 1 pouch
For surgical implantation
innocoll
Distributed by: Innocoll Pharmaceuticals Ltd. Athlone, Ireland N37 VW42
-
PRINCIPAL DISPLAY PANEL
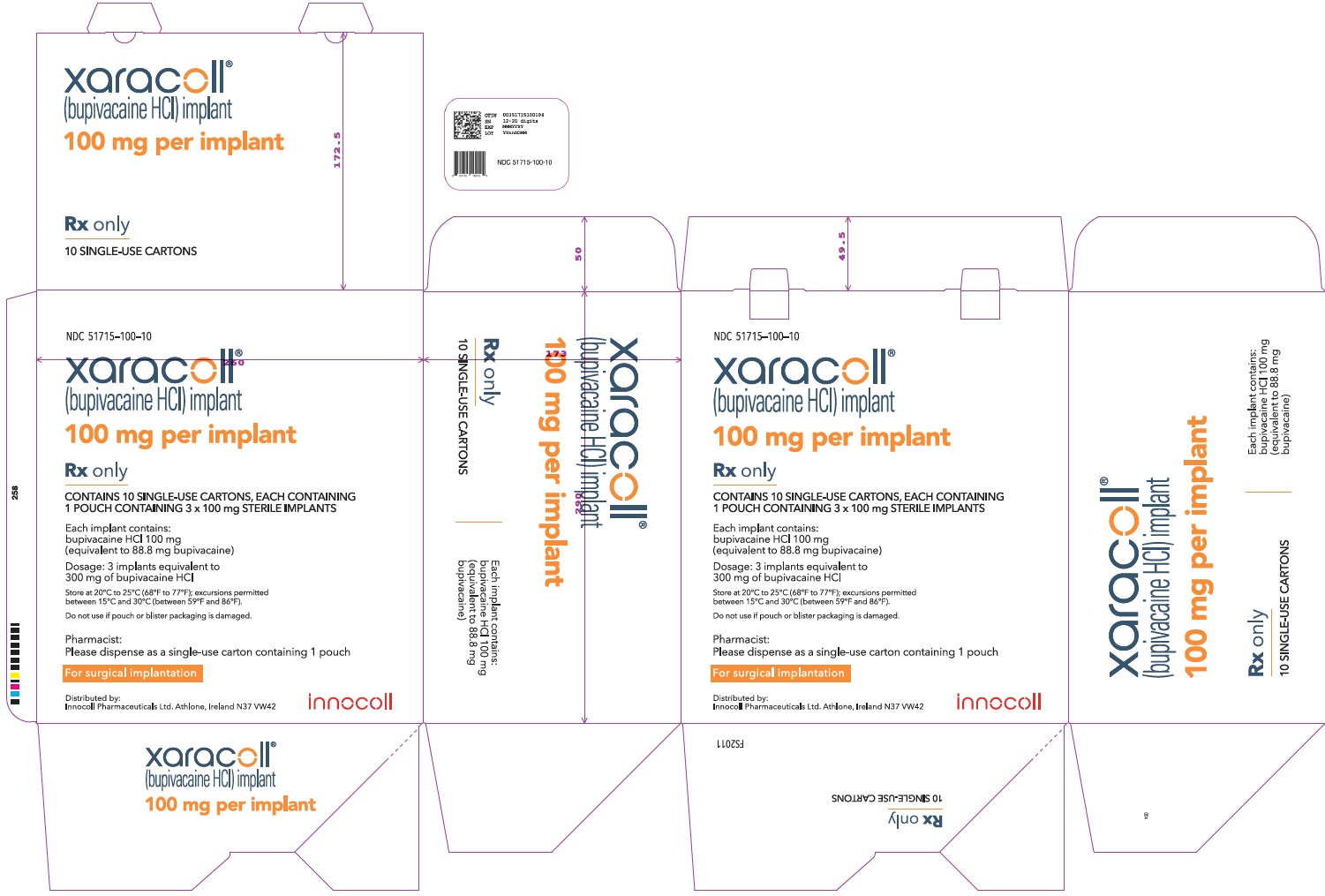
NDC 51715-100-10
xaracoll®
(bupivacaine HCl) implant
100 mg per implant
Rx only
CONTAINS 10 SINGLE-USE CARTONS, EACH CONTAINING 1 POUCH CONTAINING 3 x 100 mg STERILE IMPLANTS
Each implant contains: bupivacaine HCl 100 mg (equivalent to 88.8 mg bupivacaine)
Dosage: 3 implants equivalent to 300 mg of bupivacaine HCl
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F).
Do not use if pouch or blister packaging is damaged.
Pharmacist: Please dispense as a single-use carton containing 1 pouch
For surgical implantation
Distributed by:
Innocoll Pharmaceuticals Ltd. Athlone, Ireland N37 VW42
innocoll
-
PRINCIPAL DISPLAY PANEL
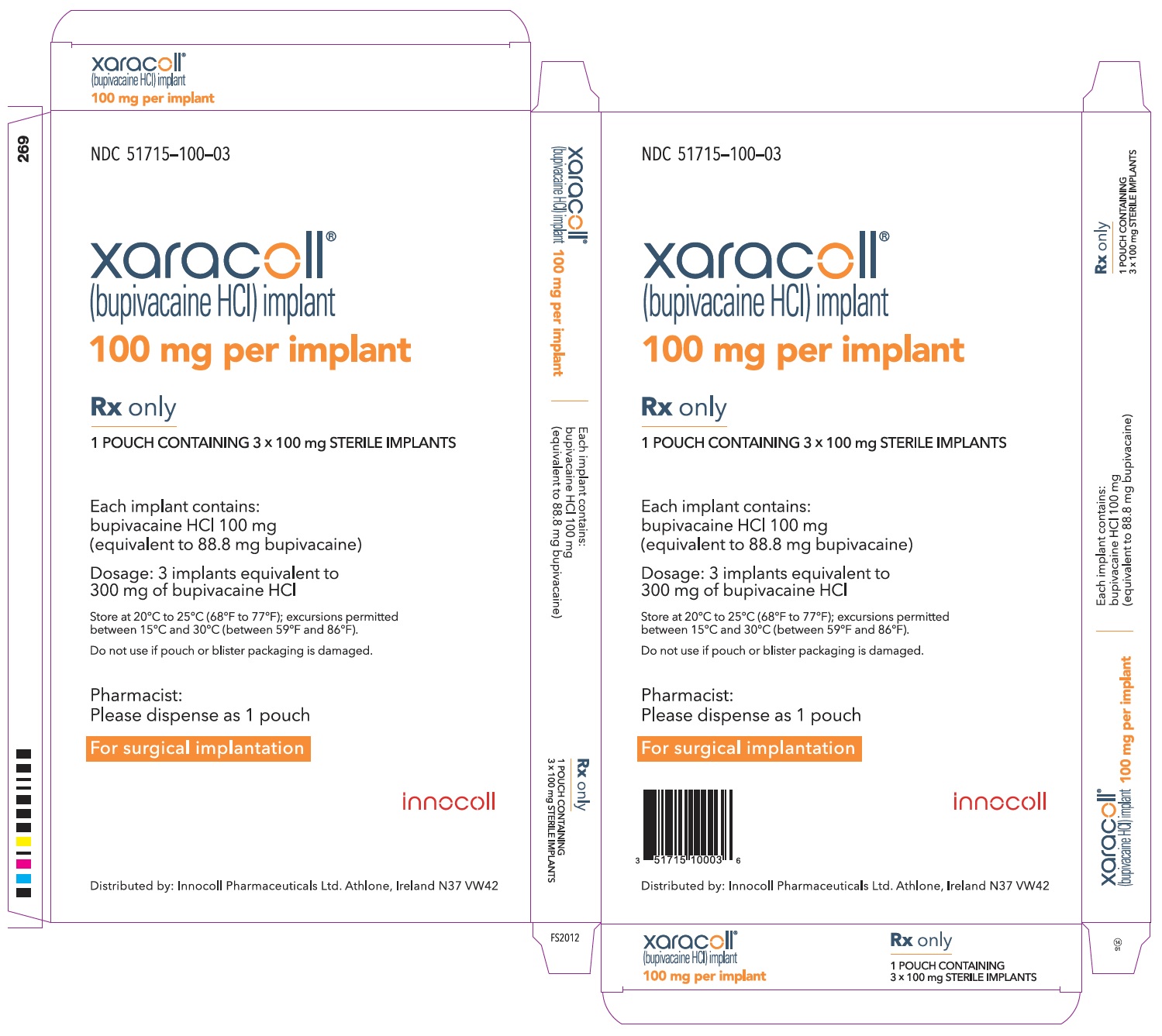
NDC 51715-100-04
xaracoll®
(bupivacaine HCl) implant
100 mg per implant
Rx only
CONTAINS 4 SINGLE-USE CARTONS, EACH CONTAINING 1 POUCH CONTAINING 3 x 100 mg STERILE IMPLANTS
Each implant contains: bupivacaine HCl 100 mg (equivalent to 88.8 mg bupivacaine)
Dosage: 3 implants equivalent to 300 mg of bupivacaine HCl
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F).
Do not use if pouch or blister packaging is damaged.
Pharmacist: Please dispense as a single-use carton containing 1 pouch
For surgical implantation
innocoll
Distributed by: Innocoll Pharmaceuticals Ltd. Athlone, Ireland N37 VW42

-
INGREDIENTS AND APPEARANCE
XARACOLL
bupivacaine hcl collagen-matrix implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51715-100 Route of Administration Parenteral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE (UNII: Y8335394RO) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE 100 mg Product Characteristics Color white (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51715-100-04 4 in 1 CARTON 08/28/2020 1 1 in 1 CARTON 1 3 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:51715-100-10 10 in 1 CARTON 08/28/2020 2 1 in 1 CARTON 2 3 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209511 08/28/2020 Labeler - INNOCOLL PHARMACEUTICALS (989921395) Registrant - Innocoll Pharmaceuticals Limited (989921395) Establishment Name Address ID/FEI Business Operations Syntacoll GmbH 318155959 manufacture(51715-100)