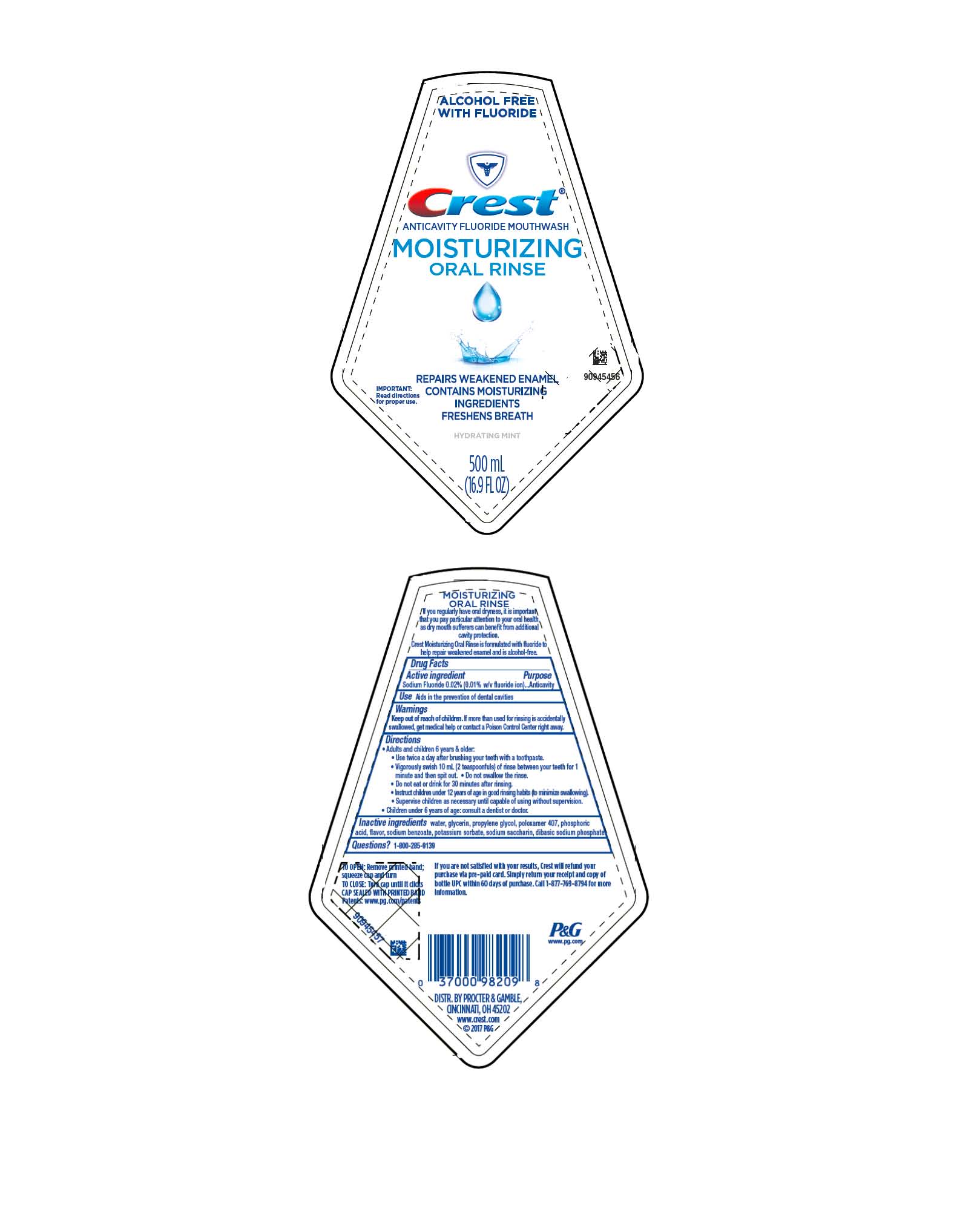
CREST MOISTURIZING- sodium fluoride rinse
The Procter & Gamble Manufacturing Company
----------
Crest
®
Moisturizing Oral Rinse
Directions
- Adults and children 6 years & older:
- Use twice a day after brushing your teeth with a toothpaste.
- Vigorously swish 10 mL (2 teaspoonfuls) of rinse between your teeth for 1 minute and then spit out.
- Do not swallow the rinse.
- Do not eat or drink for 30 minutes after rinsing.
- Instruct children under 12 years of age in good rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using without supervision.
- Children under 6 years of age: consult a dentist or doctor.
| CREST
MOISTURIZING
sodium fluoride rinse |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 10/2023
Document Id: 07fe1cee-eae5-3d4a-e063-6294a90a03be
Set id: 468c01ff-3bbb-09dd-e054-00144ff8d46c
Version: 6
Effective Time: 20231018
The Procter & Gamble Manufacturing Company