Label: HEPATITIS B SURFACE ANTIGEN- hepatitis b virus subtype adw hbsag surface protein antigen liquid
- NDC Code(s): 69806-0002-0
- Packager: Merck Sharp & Dohme Corp.
- Category: LICENSED VACCINE BULK INTERMEDIATE LABEL
Drug Label Information
Updated August 5, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
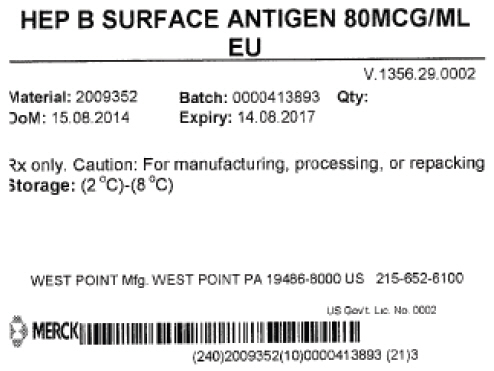
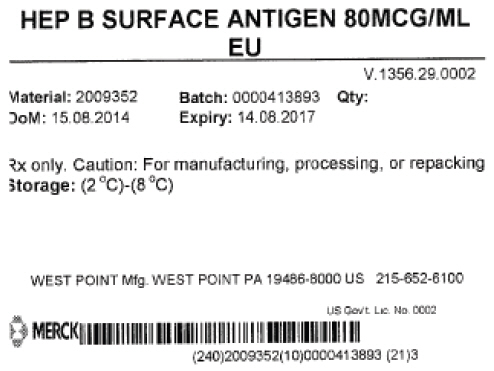
- PRINCIPAL DISPLAY PANEL - Shipping Label
-
INGREDIENTS AND APPEARANCE
HEPATITIS B SURFACE ANTIGEN
hepatitis b virus subtype adw hbsag surface protein antigen liquidProduct Information Product Type LICENSED VACCINE BULK INTERMEDIATE Item Code (Source) NDC:69806-0002 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN (UNII: XL4HLC6JH6) (HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN - UNII:XL4HLC6JH6) HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69806-0002-0 1 in 1 CONTAINER 1 37000 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 06/10/2015 Labeler - Merck Sharp & Dohme Corp. (002387926)