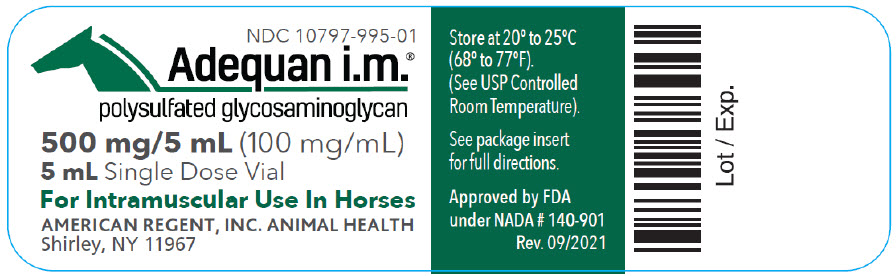
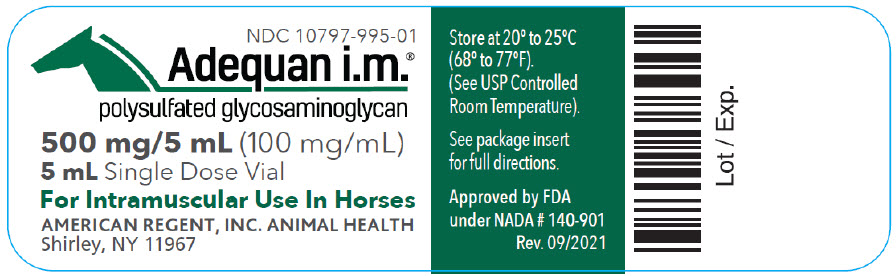
Label: ADEQUAN I.M.- polysulfated glycosaminoglycan injection, solution
- NDC Code(s): 10797-995-01, 10797-995-70
- Packager: American Regent, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION:
- DESCRIPTION:
-
PHARMACOLOGY:
Polysulfated Glycosaminoglycan is chemically similar to the glycosaminoglycans in articular cartilage matrix. PSGAG is a potent proteolytic enzyme inhibitor and diminishes or reverses the pathologic processes of traumatic or degenerative joint disease which result in a net loss of cartilage matrix components. PSGAG improves joint function by reducing synovial fluid protein levels and increasing synovial fluid hyaluronic acid concentration in traumatized equine carpal and hock joints.
- INDICATIONS:
- DOSAGE AND ADMINISTRATION:
- CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
-
ANIMAL SAFETY:
Toxicity studies were conducted in horses. Doses as high as 2,500 mg were administered intramuscularly to 6 horses twice a week for 12 weeks. This dosage is 5 times the recommended dosage and 3 times the recommended therapeutic regimen. Clinical observations revealed no soreness or swelling at the injection site or in the affected joint. No animal had any clinical or laboratory evidence of toxicity.
- STORAGE CONDITIONS:
- HOW SUPPLIED:
- Container Label
-
Carton Labeling
NDC 10797-995-70
Adequani.m.®
polysulfated glycosaminoglycan
PSGAG Injection
500 mg/5 mL (100 mg/mL)
For the intramuscular use in horses.
For the treatment of non-infectious degenerative and/or traumatic joint dysfunction and associated lameness of the carpal and hock joint in horses.
SINGLE DOSE VIALS
7 Vials (5 mL each)
AMERICAN REGENT, INC., ANIMAL HEALTH
Shirley, NY 11967
(1-888-354-4857)
Approved by FDA under NADA # 140-901
- Serialization Label
-
INGREDIENTS AND APPEARANCE
ADEQUAN I.M.
polysulfated glycosaminoglycan injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:10797-995 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYSULFATED GLYCOSAMINOGLYCAN (UNII: 268AW7000T) (POLYSULFATED GLYCOSAMINOGLYCAN - UNII:268AW7000T) POLYSULFATED GLYCOSAMINOGLYCAN 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10797-995-70 7 in 1 CARTON 1 NDC:10797-995-01 5 mL in 1 VIAL, SINGLE-USE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140901 11/01/1989 Labeler - American Regent, Inc. (002033710) Establishment Name Address ID/FEI Business Operations American Regent, Inc. 002033710 analysis, manufacture Establishment Name Address ID/FEI Business Operations Daiichi Sankyo Altrich Sari 262417595 api manufacture