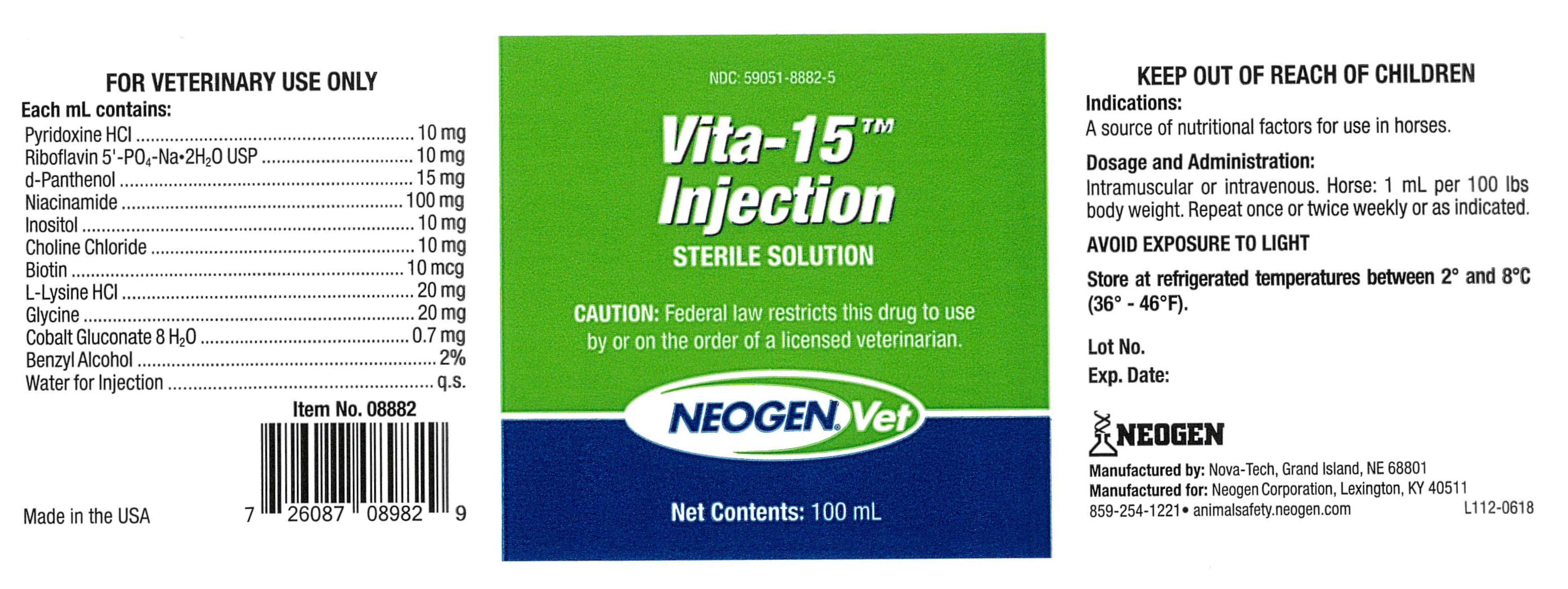
VITA-15- vita-15 injection
Neogen Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
NeogenVet
Vita-15TM
Injection
Sterile Solution
Each mL contains:
Pyridoxine HCl ...................................................... 10 mg
Riboflavin 5'-PO4-Na•2H2O USP .............................. 10 mg
d-Panthenol ......................................................... 15 mg
Niacinamide ....................................................... 100 mg
Inositol ............................................................... 10 mg
Choline Chloride ................................................... 10 mg
Biotin .................................................................. 10 mcg
L-Lysine HCl ......................................................... 20 mg
Glycine ................................................................ 20 mg
Cobalt Gluconate 8 H2O ......................................... 0.7 mg
Benzyl Alcohol ........................................................... 2%
Water for Injection .................................................... q.s.
Dosage & Administration:
Intramuscular or intravenous. Horse: 1 mL per 100 lbs
body weight. Repeat once or twice weekly or as indicated.
CAUTION:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR VETERINARY USE ONLY
KEEP OUT OF REACH OF CHILDREN
| VITA-15
vita-15 injection |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Neogen Corporation (042125879) |
| Registrant - Nova-Tech, Inc (196078976) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nova-Tech, Inc | 196078976 | manufacture | |