SHISEIDO PERFECT REFINING FOUNDATION I00- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION I20- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION I40- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION I60- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION I100- octinoxate and titanium dioxide cream
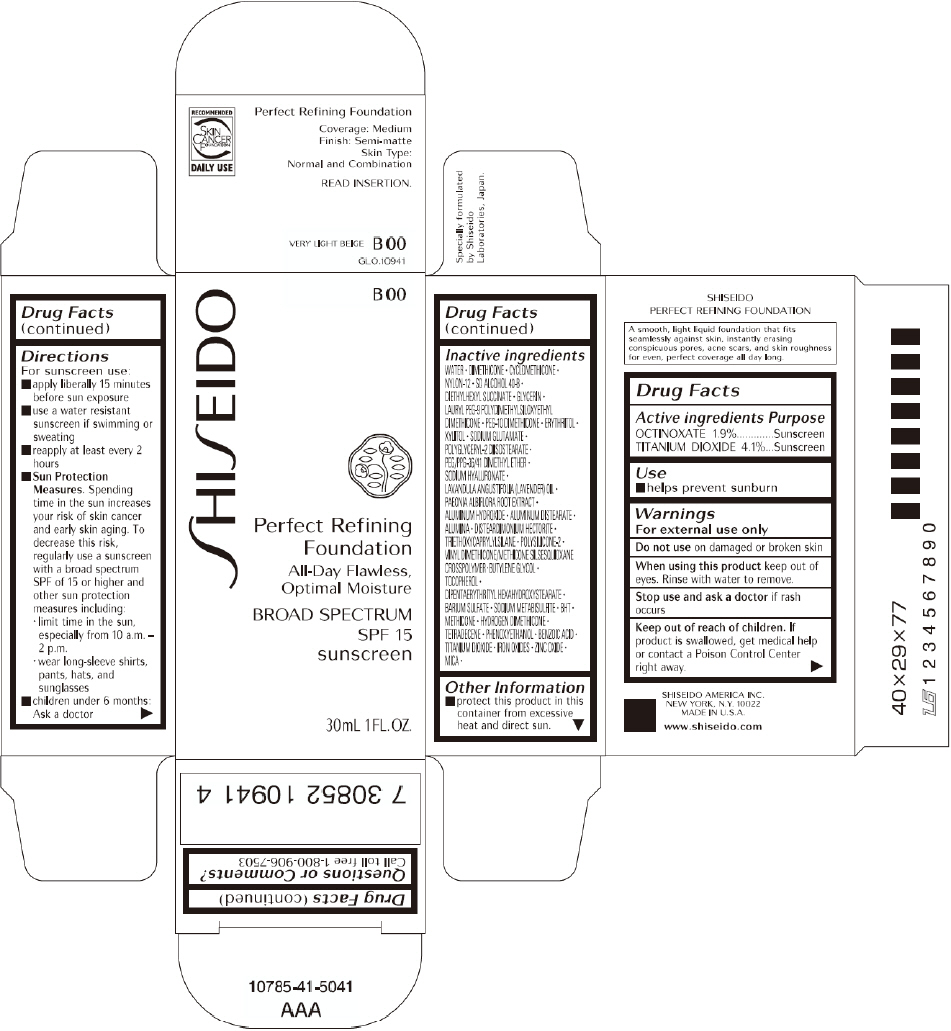
SHISEIDO PERFECT REFINING FOUNDATION B00- octinoxate and titanium dioxide cream
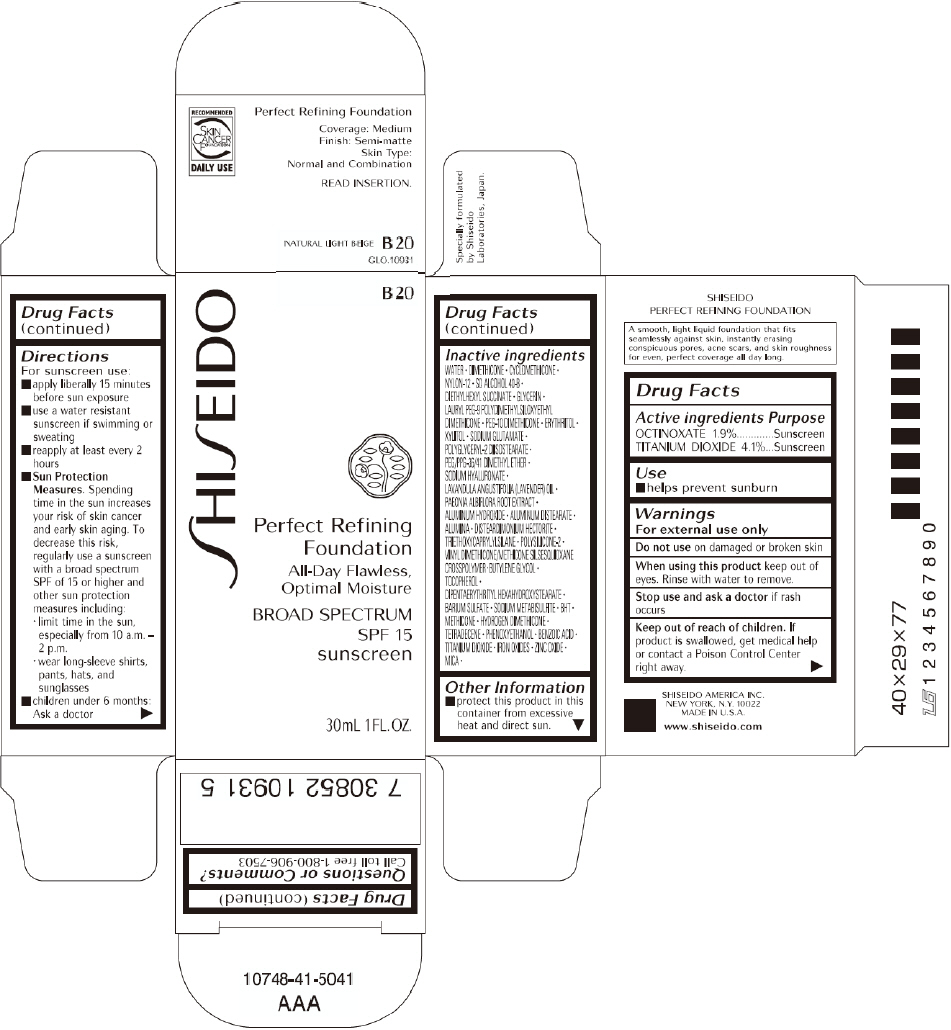
SHISEIDO PERFECT REFINING FOUNDATION B20- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION B40- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION B60- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION B100- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION O00- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION O20- octinoxate and titanium dioxide cream
SHISEIDO PERFECT REFINING FOUNDATION O40- octinoxate and titanium dioxide cream
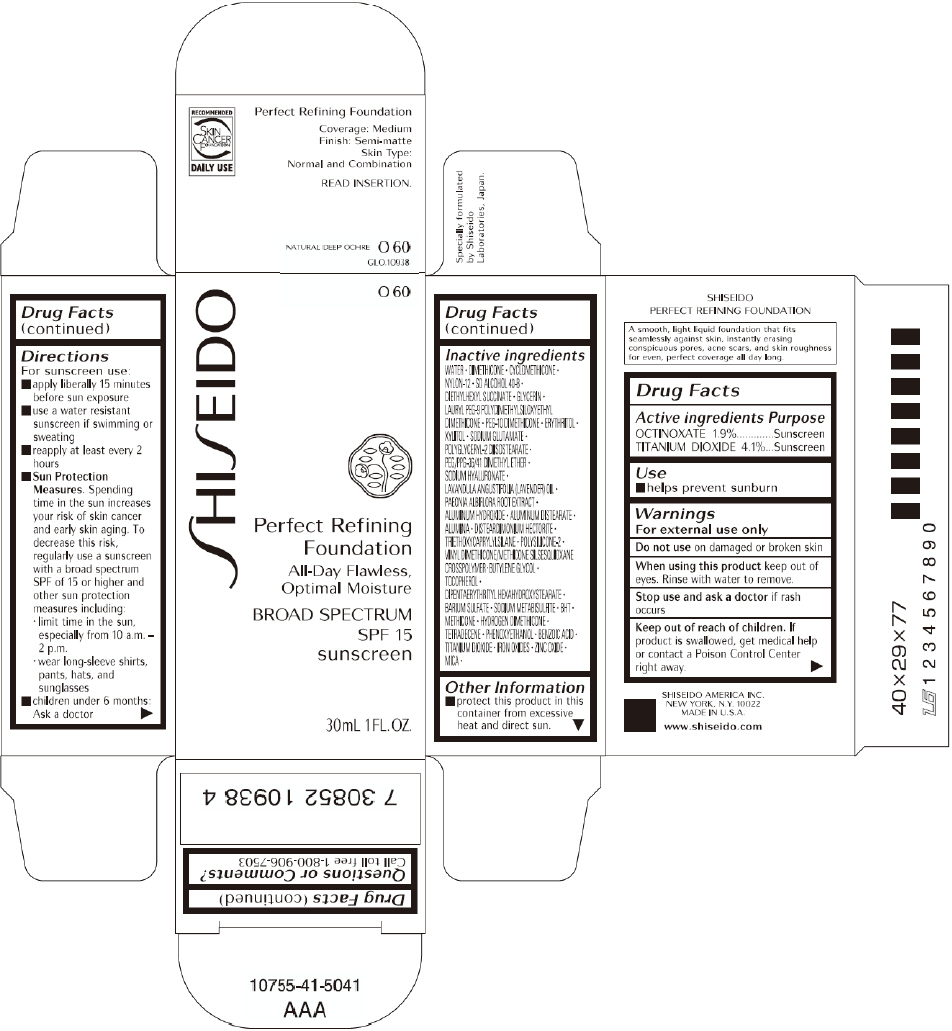
SHISEIDO PERFECT REFINING FOUNDATION O60- octinoxate and titanium dioxide cream
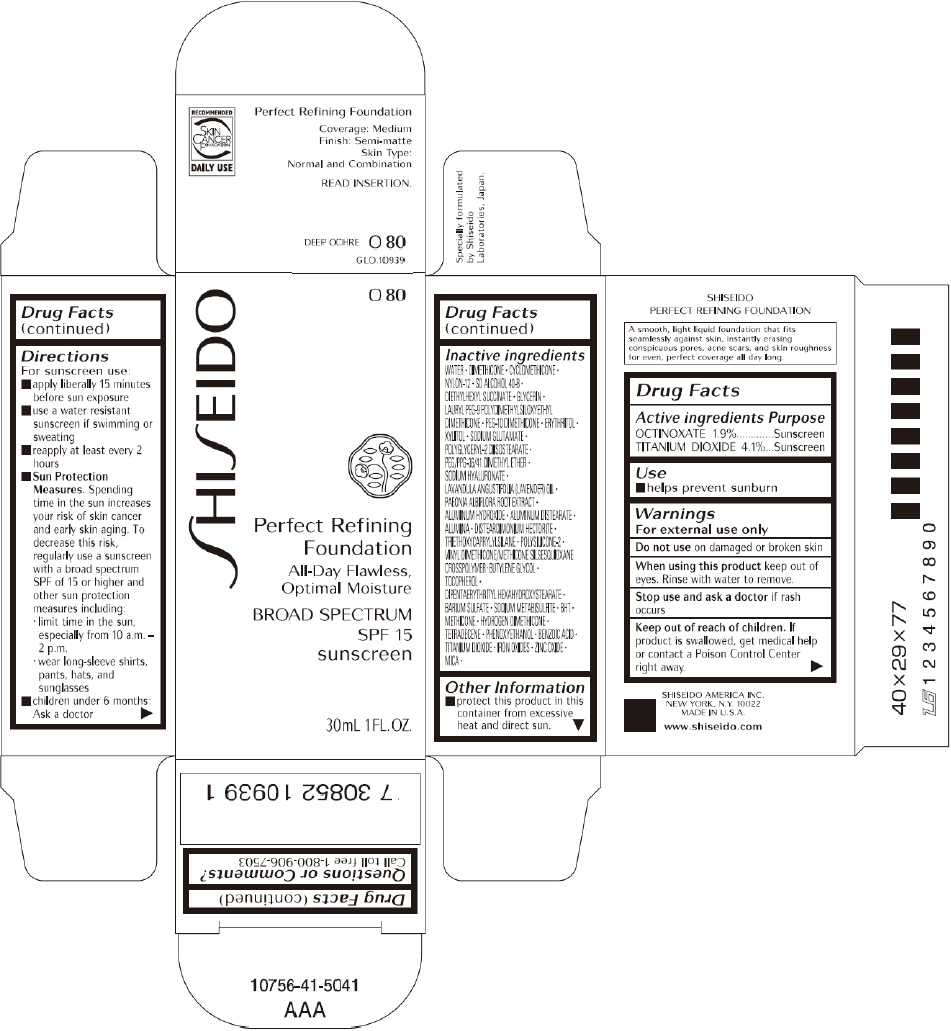
SHISEIDO PERFECT REFINING FOUNDATION O80- octinoxate and titanium dioxide cream
SHISEIDO AMERICA INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SHISEIDO PERFECT REFINING FOUNDATION
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER, DIMETHICONE, CYCLOMETHICONE, NYLON-12, SD ALCOHOL 40-B, DIETHYLHEXYL SUCCINATE, GLYCERIN, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, PEG-10 DIMETHICONE, ERYTHRITOL, XYLITOL, SODIUM GLUTAMATE, POLYGLYCERYL-2 DIISOSTEARATE, PEG/PPG-36/41 DIMETHYL ETHER, SODIUM HYALURONATE, LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL, PAEONIA ALBIFLORA ROOT EXTRACT, ALUMINUM HYDROXIDE, ALUMINUM DISTEARATE, ALUMINA, DISTEARDIMONIUM HECTORITE, TRIETHOXYCAPRYLYLSILANE, POLYSILICONE-2, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, BUTYLENE GLYCOL, TOCOPHEROL, DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE, BARIUM SULFATE, SODIUM METABISULFITE, BHT, METHICONE, HYDROGEN DIMETHICONE, TETRADECENE, PHENOXYETHANOL, BENZOIC ACID, TITANIUM DIOXIDE, IRON OXIDES, ZINC OXIDE, MICA,
PRINCIPAL DISPLAY PANEL - 30mL B 00 Tube Carton
SHISEIDO
B 00
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
PRINCIPAL DISPLAY PANEL - 30mL B 20 Tube Carton
SHISEIDO
B 20
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
PRINCIPAL DISPLAY PANEL - 30mL B 40 Tube Carton
SHISEIDO
B 40
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
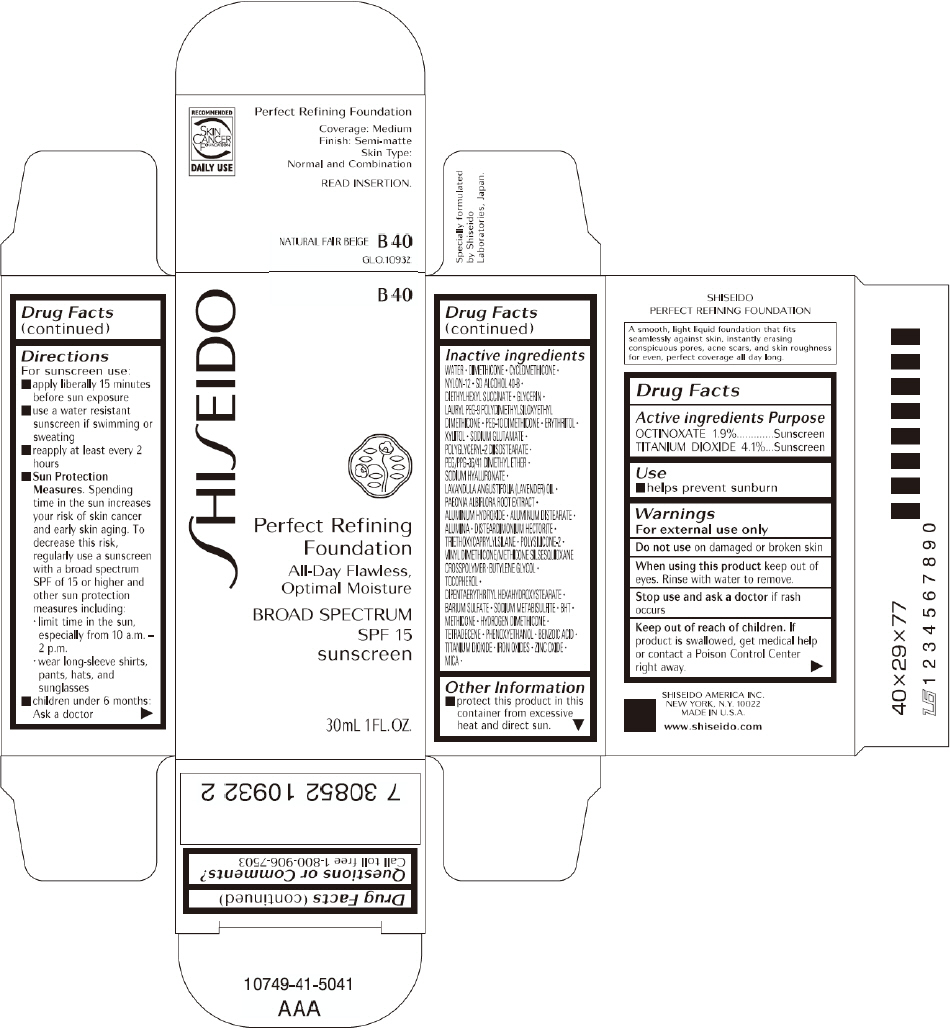
PRINCIPAL DISPLAY PANEL - 30mL B 60 Tube Carton
SHISEIDO
B 60
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
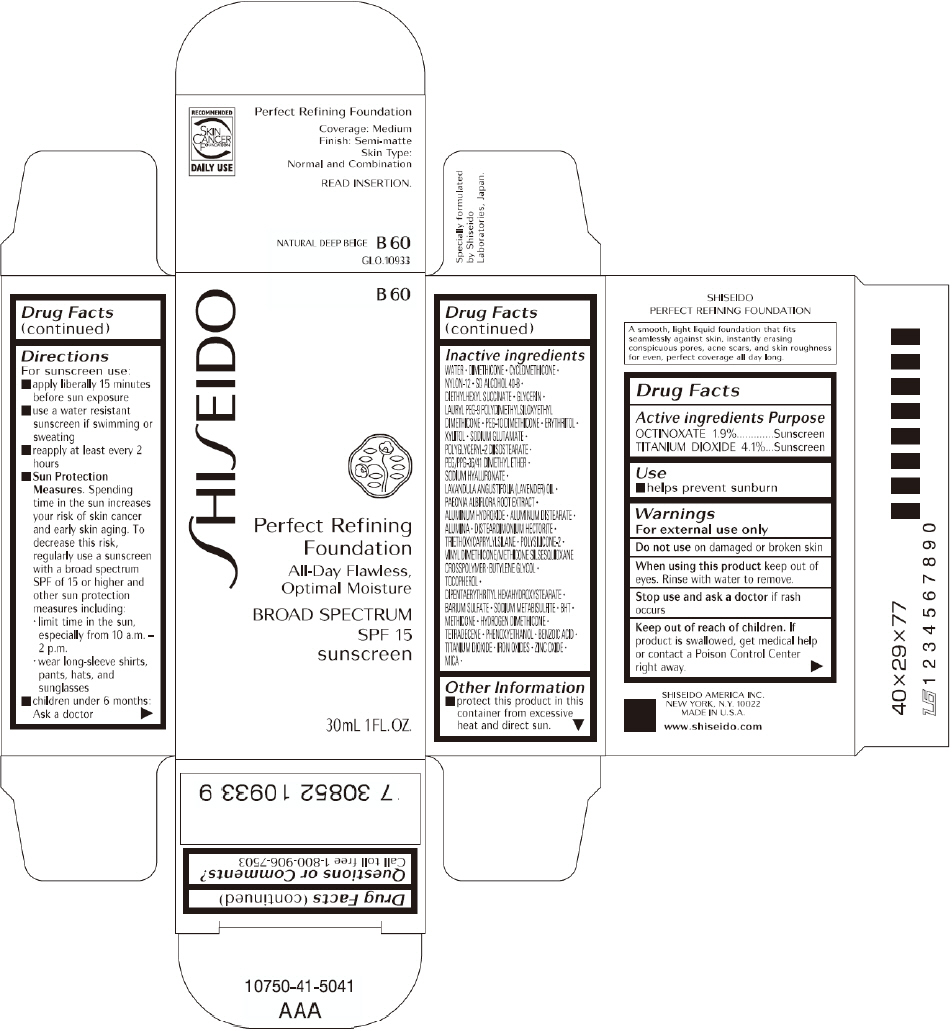
PRINCIPAL DISPLAY PANEL - 30mL B 100 Tube Carton
SHISEIDO
B 100
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
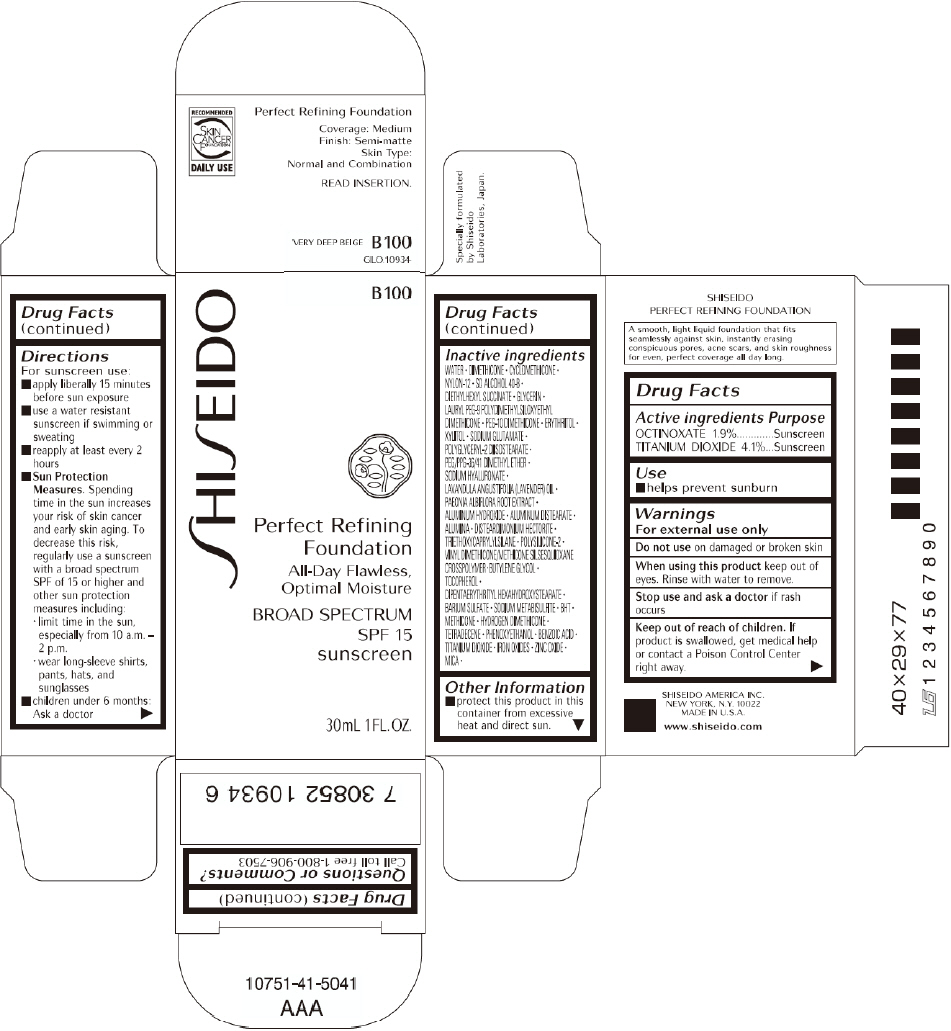
PRINCIPAL DISPLAY PANEL - 30mL I 00 Tube Carton
SHISEIDO
I 00
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
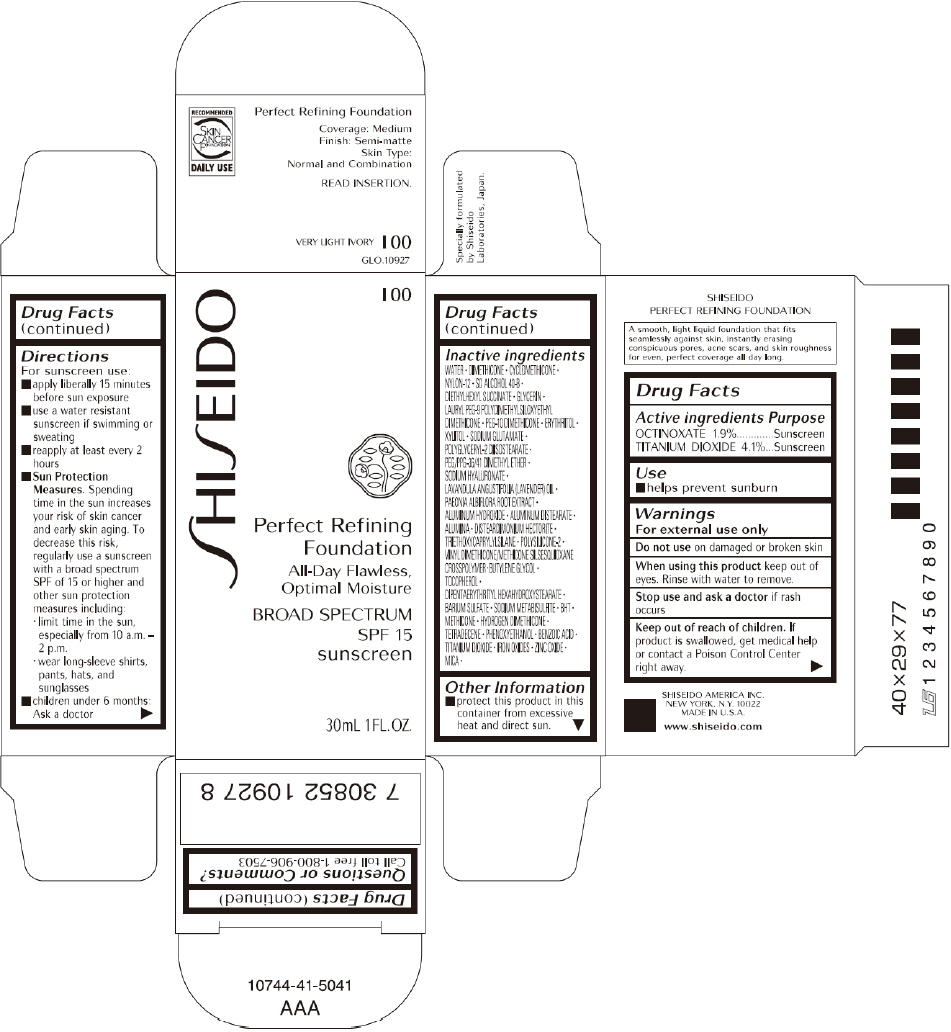
PRINCIPAL DISPLAY PANEL - 30mL I 20 Tube Carton
SHISEIDO
I 20
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
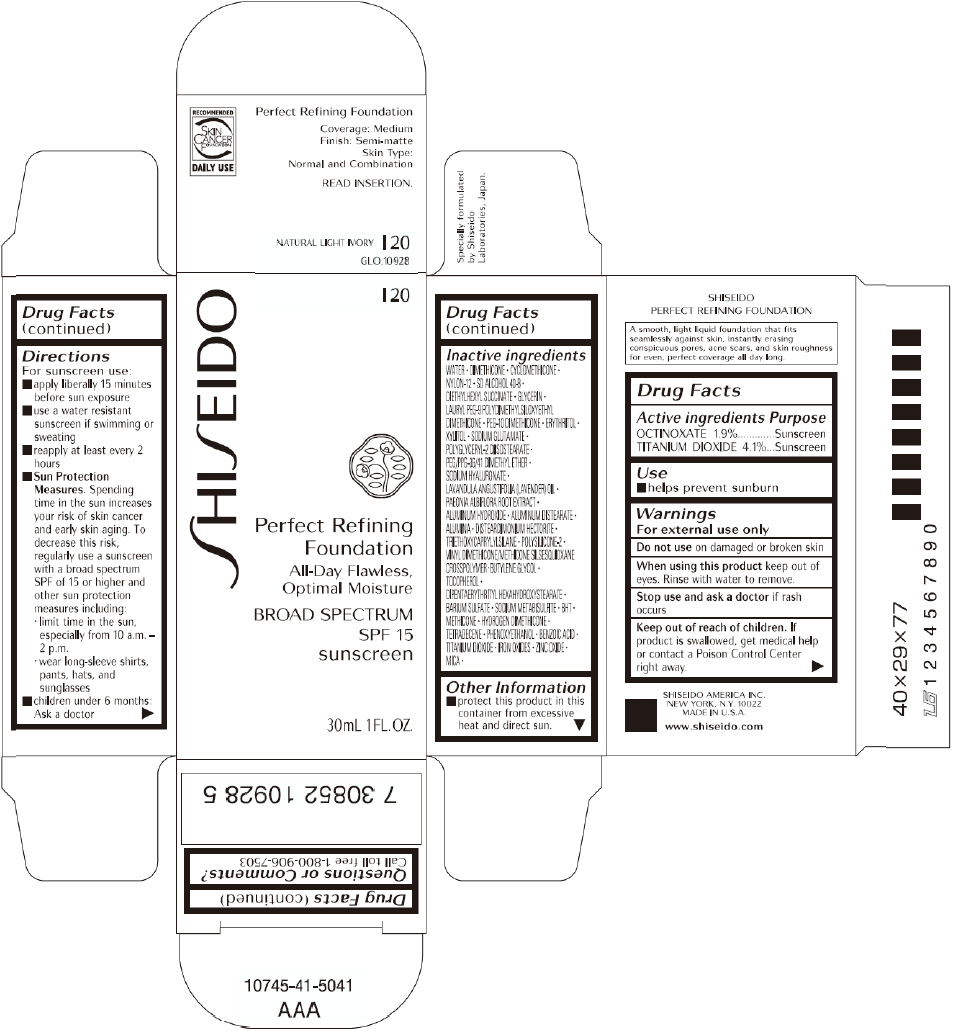
PRINCIPAL DISPLAY PANEL - 30mL I 40 Tube Carton
SHISEIDO
I 40
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
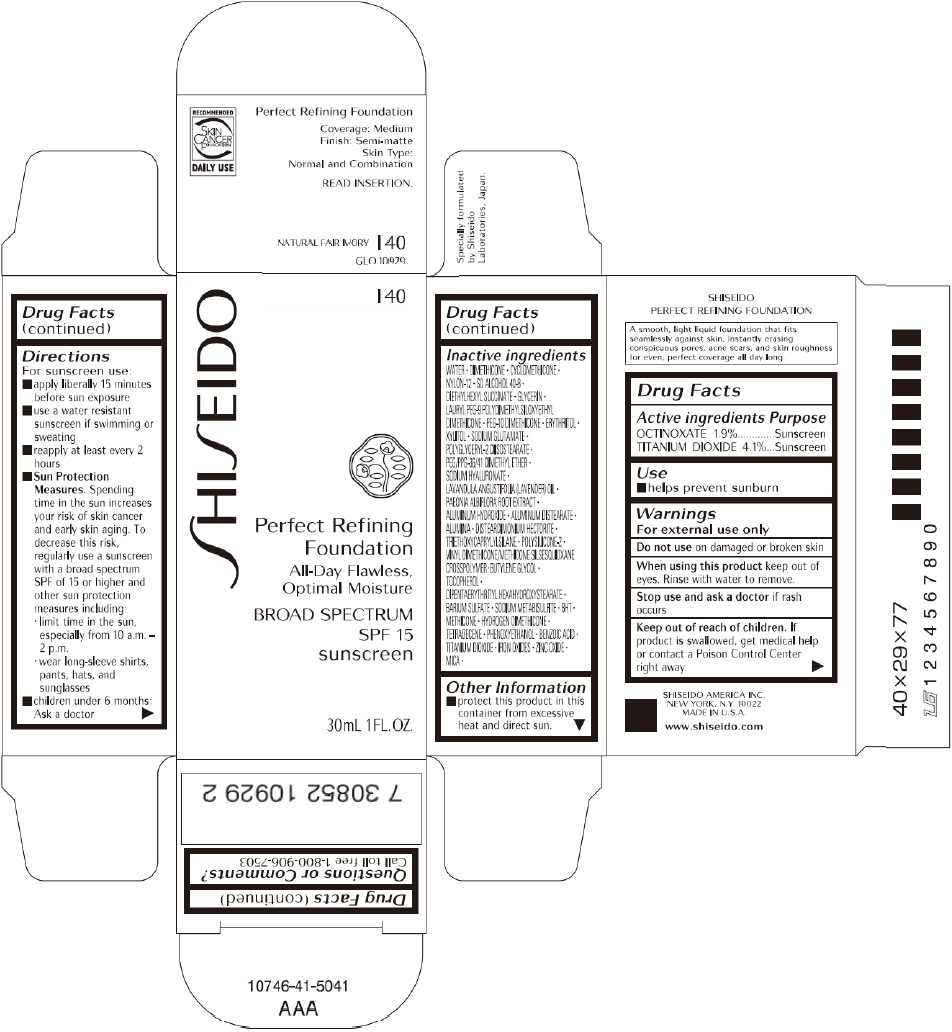
PRINCIPAL DISPLAY PANEL - 30mL I 60 Tube Carton
SHISEIDO
I 60
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
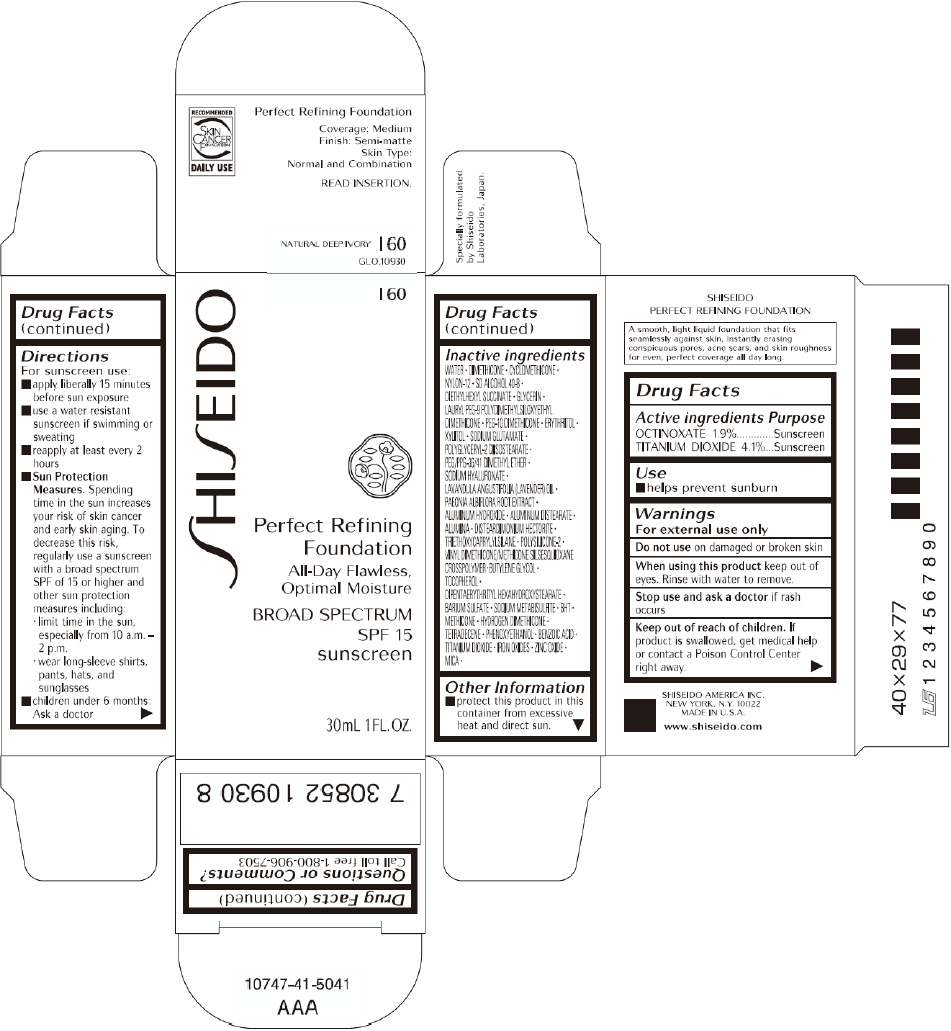
PRINCIPAL DISPLAY PANEL - 30mL I 100 Tube Carton
SHISEIDO
I 100
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
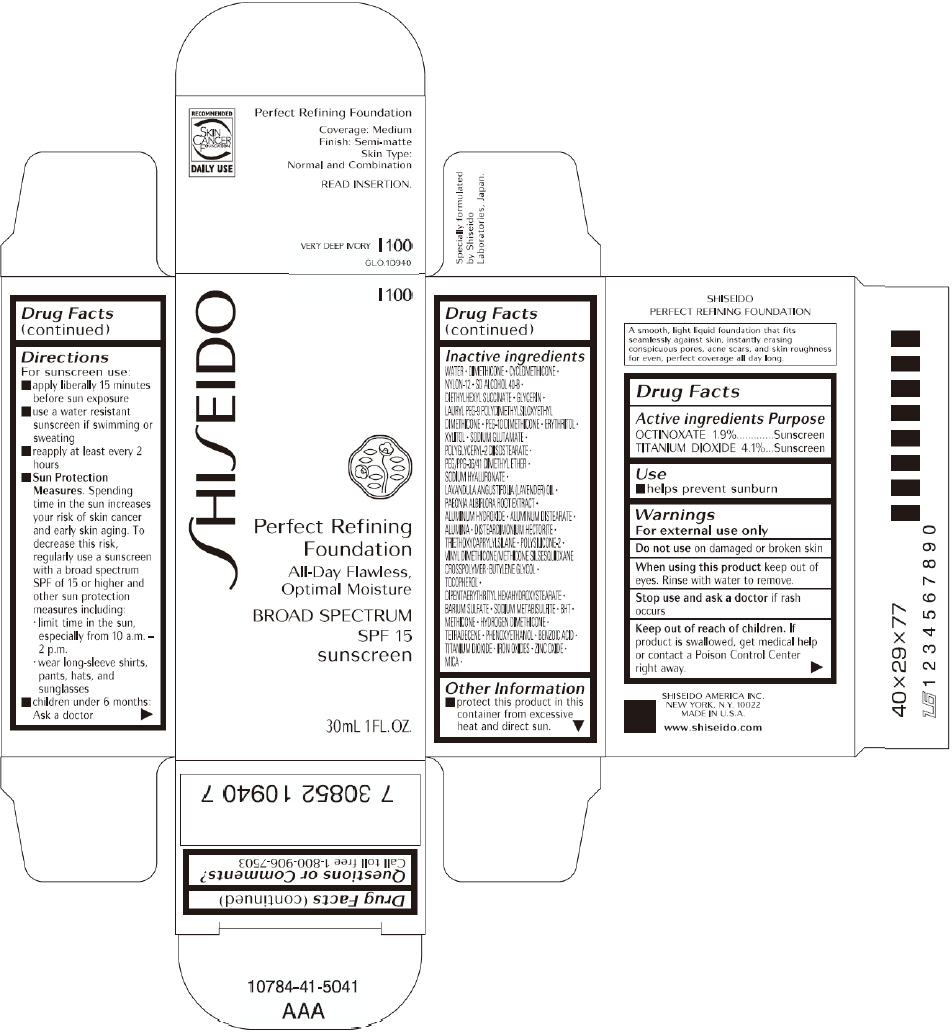
PRINCIPAL DISPLAY PANEL - 30mL O 00 Tube Carton
SHISEIDO
O 00
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
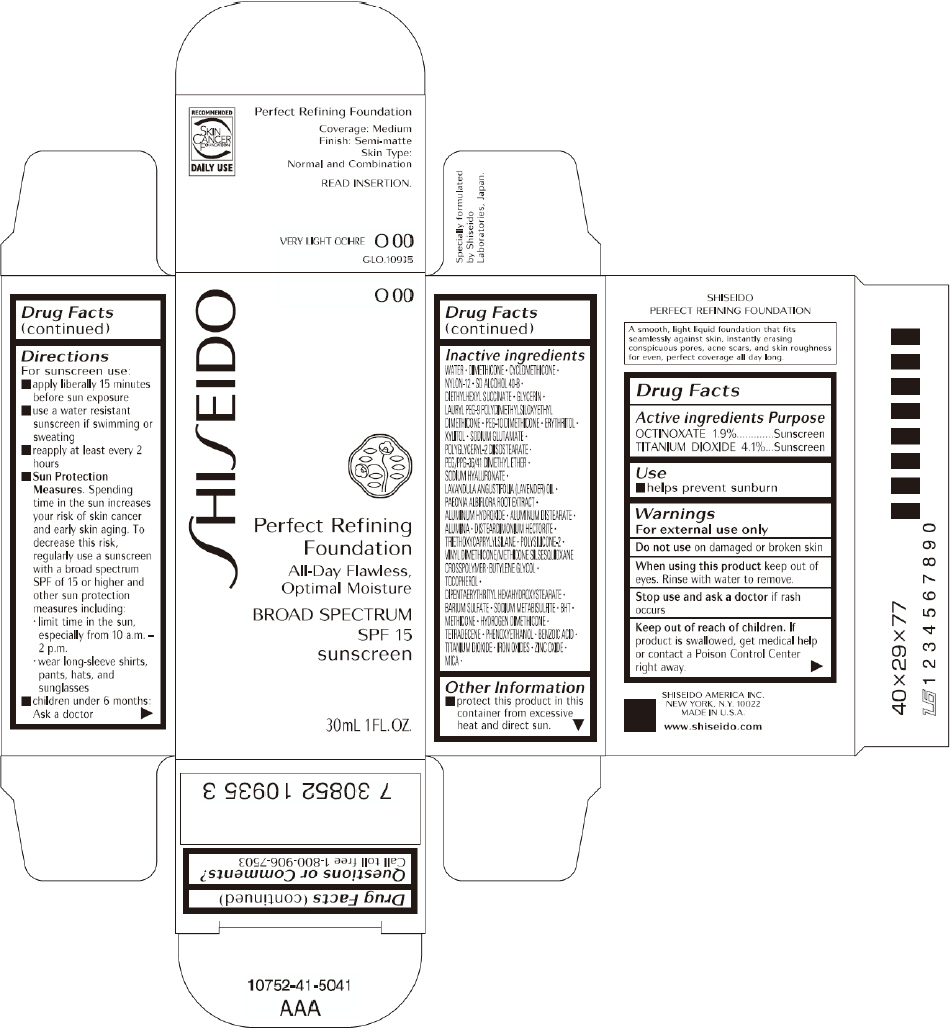
PRINCIPAL DISPLAY PANEL - 30mL O 20 Tube Carton
SHISEIDO
O 20
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
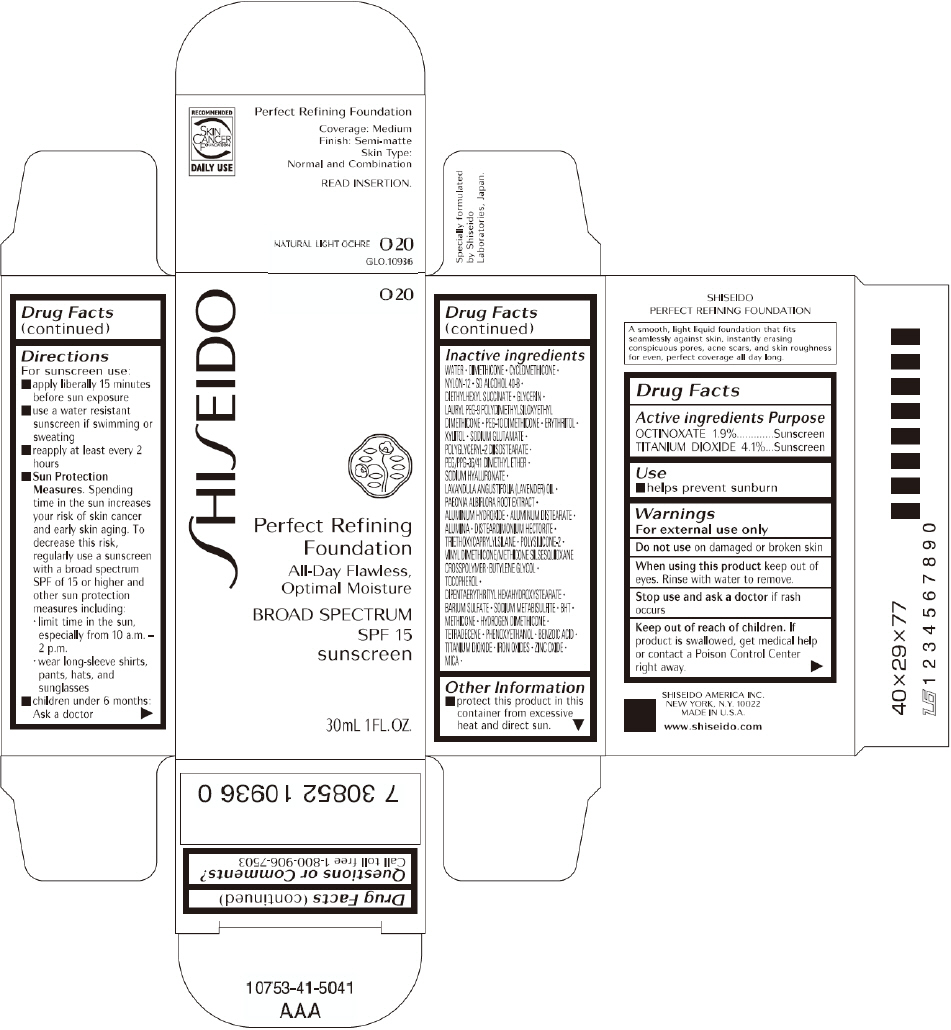
PRINCIPAL DISPLAY PANEL - 30mL O 40 Tube Carton
SHISEIDO
O 40
Perfect Refining
Foundation
All-Day Flawless,
Optimal Moisture
BROAD SPECTRUM
SPF 15
sunscreen
30mL 1FL. OZ.
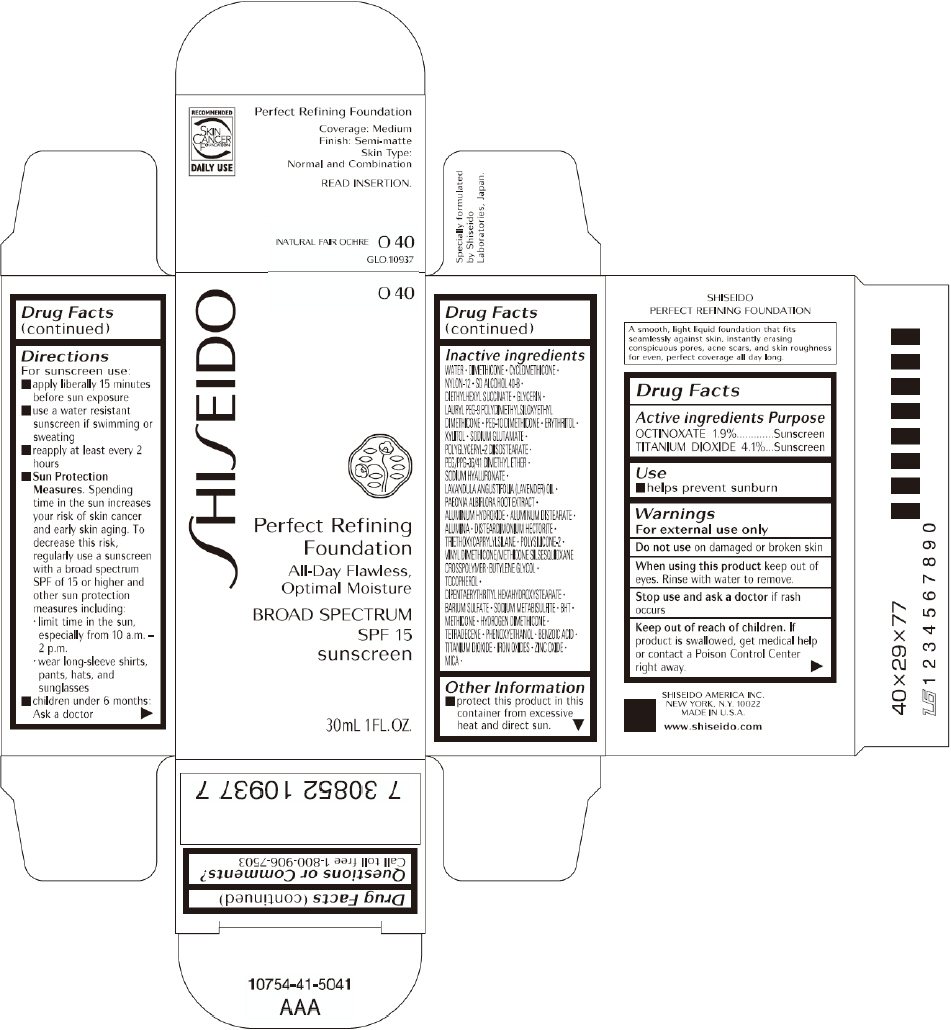
| SHISEIDO PERFECT REFINING FOUNDATION
I00
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
I20
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
I40
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
I60
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
I100
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
B00
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
B20
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
B40
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
B60
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
B100
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
O00
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
O20
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
O40
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
O60
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHISEIDO PERFECT REFINING FOUNDATION
O80
octinoxate and titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SHISEIDO AMERICA INC. (782677132) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SHISEIDO AMERICA INC. | 782677132 | manufacture(52686-232, 52686-233, 52686-234, 52686-235, 52686-236, 52686-237, 52686-238, 52686-239, 52686-240, 52686-241, 52686-242, 52686-243, 52686-244, 52686-245, 52686-246) , analysis(52686-232, 52686-233, 52686-234, 52686-235, 52686-236, 52686-237, 52686-238, 52686-239, 52686-240, 52686-241, 52686-242, 52686-243, 52686-244, 52686-245, 52686-246) | |