GAPEAM BUDIBAC- amantadine hcl, baclofen, bupivacaine hcl, cyclobenzaprine hcl, diclofenac sodium, gabapentin, and pentoxifylline
Alvix Laboratories, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Alvix-Gapeam Budibac Compounding Ingredients
For Pharmacy Prescription Compounding Only / Rx Only
For Topical Use Only
FOR PRESCRIPTION COMPOUNDING ONLY
DESCRIPTION
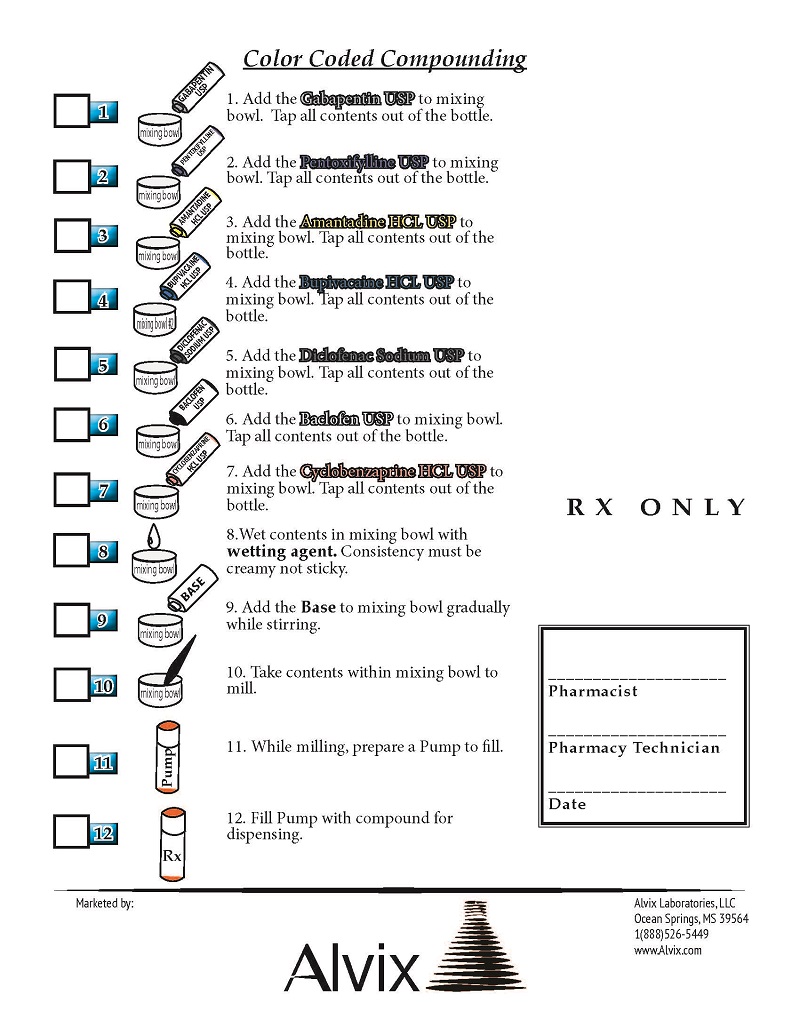
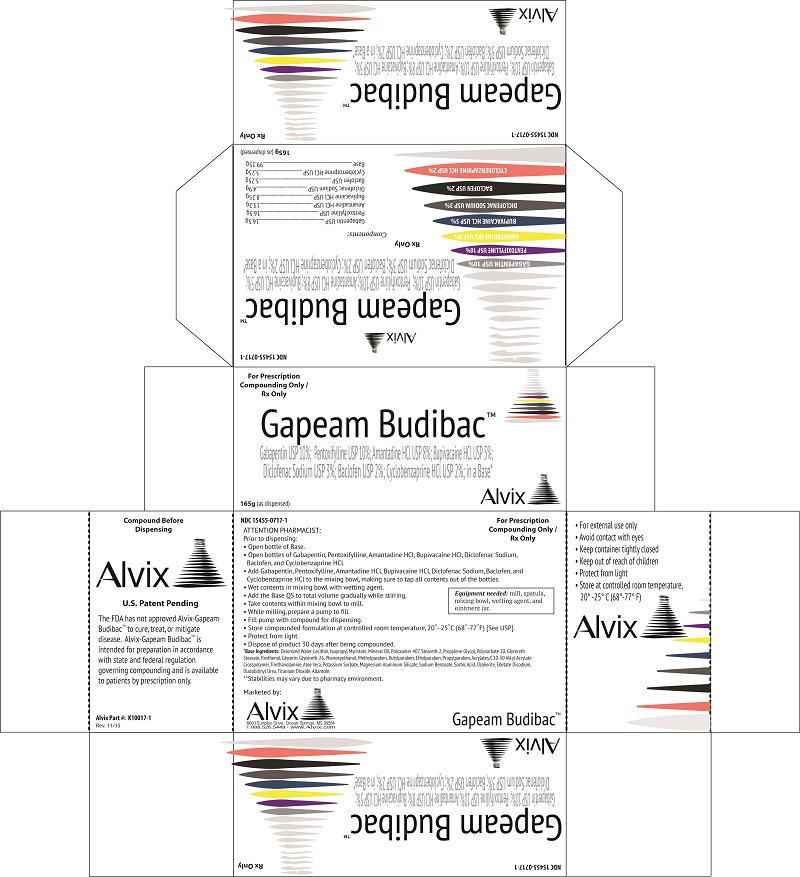
Gabapentin, Pentoxifylline, AmantadineHCL, Bupivacaine HCL, Diclofenac Sodium, Baclofen, Cyclobenzaprine HCL.
Each Alvix- Gapeam Budibac Compounding Ingredients provides 16.5 grams of Gabapentin USP, 16.5 grams of Pentoxifylline USP, 13.2 grams of Amantadine HCL USP, 8.25 grams of Bupivacaine HCL USP, 4.9 grams of Diclofenac Sodium USP, 3.25 grams of Baclofen USP, 3.25 grams of Cyclobenzaprine HCL USP for incorporation into 99.15 grams of TDC Max Cream Base.
STORAGE AND HANDLING
Prior to compounding, store Alvix- Gapeam Budibac Compounding Ingredients at room temperature between 15 - 30 degrees C (59 - 86 degrees F).
For non-sterile use only. Avoid contact with eyes. Keep container tightly closed. Keep out of reach of children. Protect from light. Dispose of product after 30 days of being compounded.
| GAPEAM BUDIBAC
amantadine hcl, baclofen, bupivacaine hcl, cyclobenzaprine hcl, diclofenac sodium, gabapentin, and pentoxifylline kit |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Alvix Laboratories, LLC (962445925) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alvix Laboratories, LLC | 962445925 | pack(15455-0717) | |