NICH PVPI UREA
- povidone-iodine for solution
Nich Marketers, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
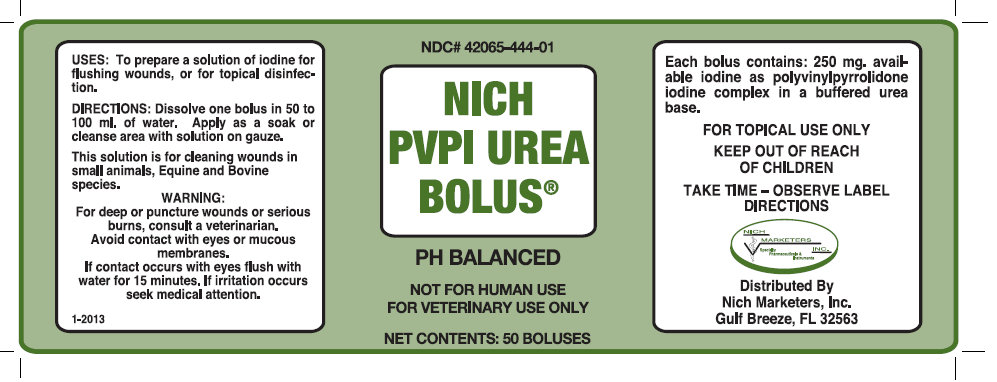
NICH PVPI UREA BOLUS
DIRECTIONS: Dissolve one bolus in 50 to 100 ml of water. Apply as a soak or cleanse area with solution on gauze.
This solutions is for cleaning wounds in small animals, Equine and Bovine species.
WARNING:
For deep or puncture wounds or serious burns, consult a veterinarian.
Avoid contact with eyes or mucous membranes.
If contact occurs with eyes flush with water for 15 minutes. If irritation occurs seek medical attention.
| NICH PVPI UREA
povidone-iodine for solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Nich Marketers, Inc (883363012) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Performance Products, Inc | 006265888 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Marcus Research Laboratory, Inc. | 075910356 | api manufacture | |
Revised: 3/2024
Document Id: 5079afd6-3895-4194-9d81-b118d565abf1
Set id: 43b59176-73a2-4e64-a836-f6297505f353
Version: 11
Effective Time: 20240314
Nich Marketers, Inc