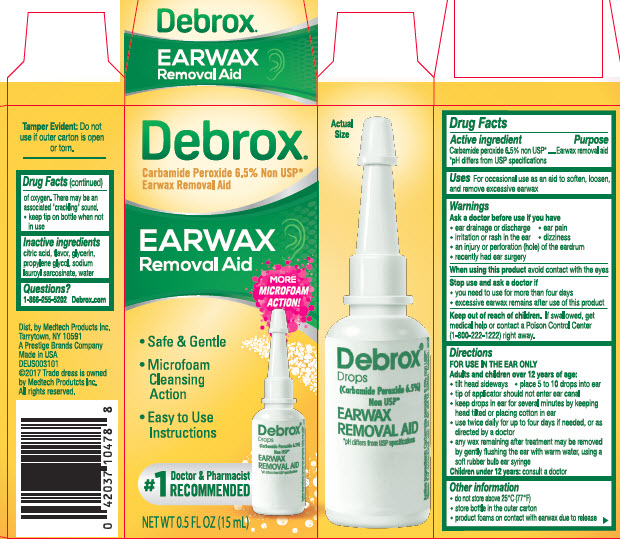
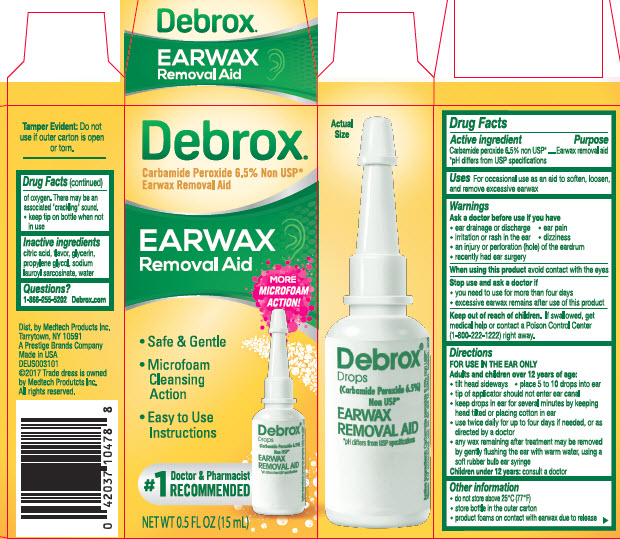
Label: DEBROX- carbamide peroxide liquid
- NDC Code(s): 63029-321-01
- Packager: Medtech Products Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 5, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep this and all drugs out of the reach of children.
-
Directions
FOR USE IN THE EAR ONLY
adults and children over 12 years of age:- tilt head sideways
- place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by keeping head tilted or placing cotton in ear
- use twice daily for up to four days if needed, or as directed by a doctor
- any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
children under 12 years: consult a doctor
- Other information
- Inactive Ingredients
- Questions?
- Debrox Earwax Removal Aid 0.5 FL OZ (15 mL)
-
INGREDIENTS AND APPEARANCE
DEBROX
carbamide peroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63029-321 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 0.065 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63029-321-01 1 in 1 CARTON 08/01/2012 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part344 08/01/2012 Labeler - Medtech Products Inc. (122715688)