BIOCELL- lactic acid liquid
DeLaval Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DeLaval
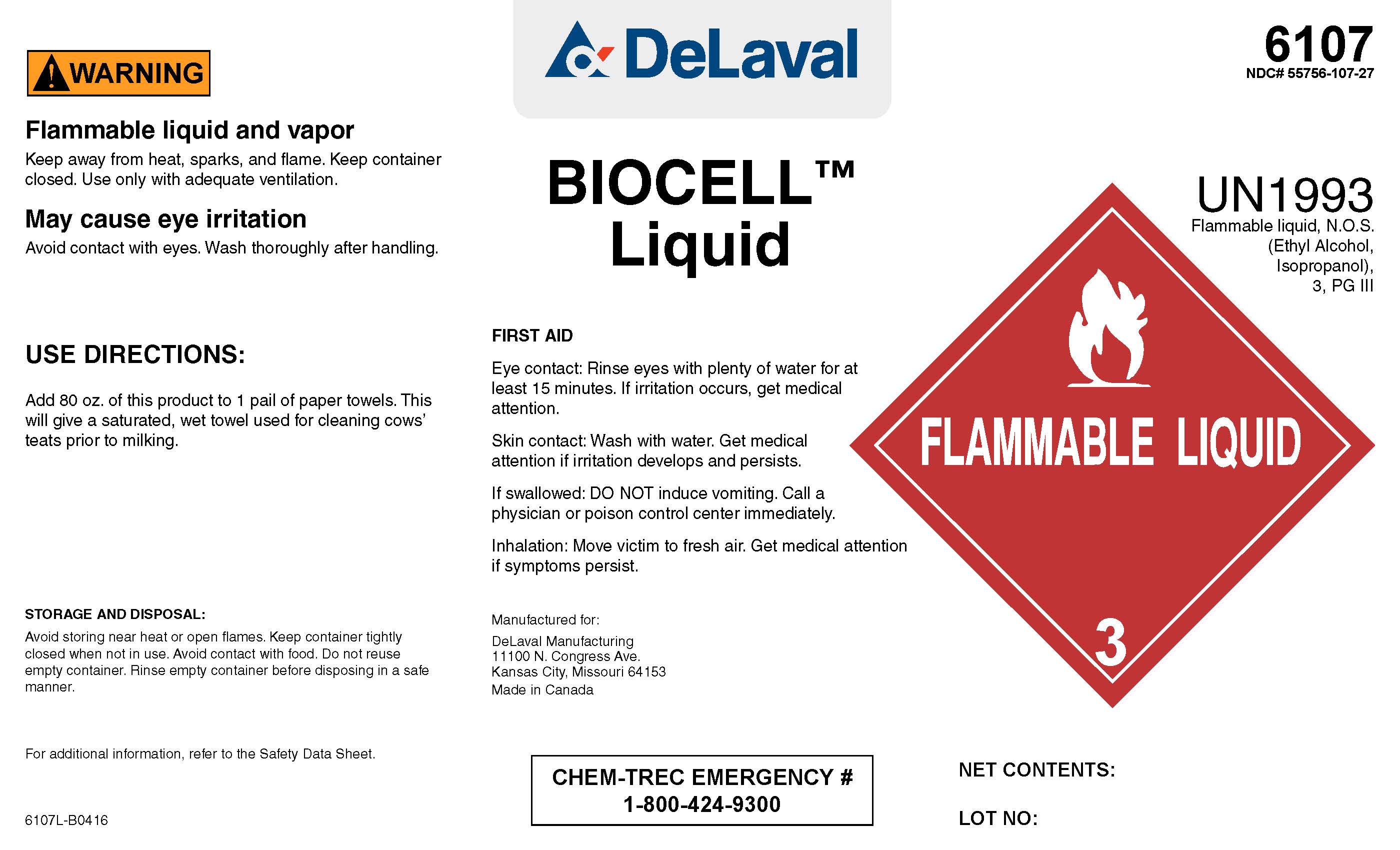
BIOCELL Liquid
! WARNING
Flammable liquid and vapor
Keep away from heat, sparks, and flame. Keep container closed. Use only with adequate ventilation.
May cause eye irritation
Avoid contact with eyes. Wash thoroughly after handling.
USE DIRECTIONS:
Add 80 oz. of this product to 1 pail of paper towels. This will give a saturated, wet towel for cleaning cows' teats prior to milking.
STORAGE AND DISPOSAL:
Avoid storing near heat or open flames. Keep container tightly closed when not in use. Avoid contact with food. Do not reuse empty container. Rinse empty container before disposing in a safe manner.
For additional information, refer to the Safety Data Sheet.
FIRST AID
Eye contact: Rinse eyes with plenty of water for at least 15 minutes. If irritation occurs, get medical attention.
Skin cntact: Wash with water. Get medical attention if irritation develops and persists.
If swallowed: DO NOT induce vomiting. Call a physician or poison control center immediately.
Inhalation: Move victim to fresh air. Get medical attention if symptoms persist.
| BIOCELL
lactic acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - DeLaval Inc. (173704867) |
| Registrant - DeLaval Inc. (173704867) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West Penetone Inc. | 202373015 | analysis, api manufacture, label, manufacture, pack | |