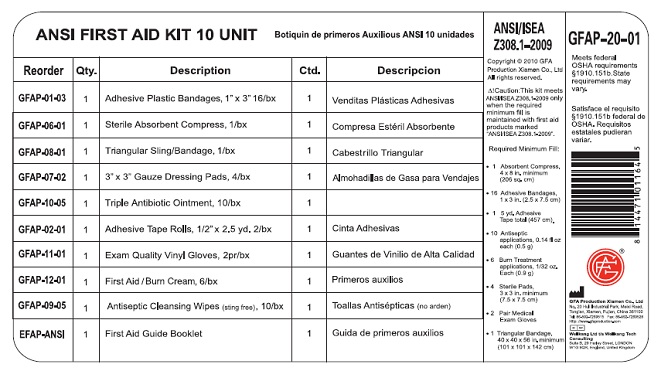
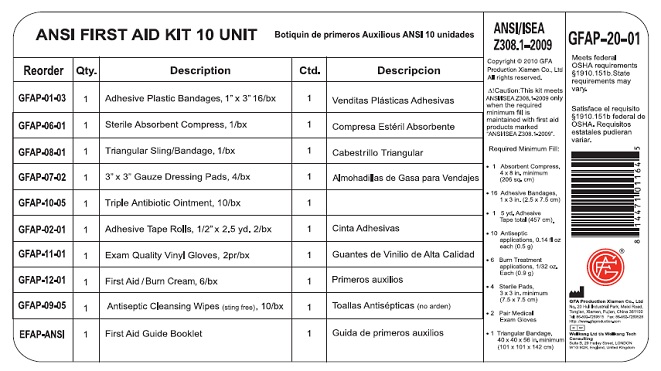
Label: GFA FIRST AID- benzalkonium chloride, benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 52124-0001-2, 52124-0003-2, 52124-0004-2, 52124-1164-3 - Packager: Genuine First Aid, LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 13, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DO NOT USE
- Directions
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
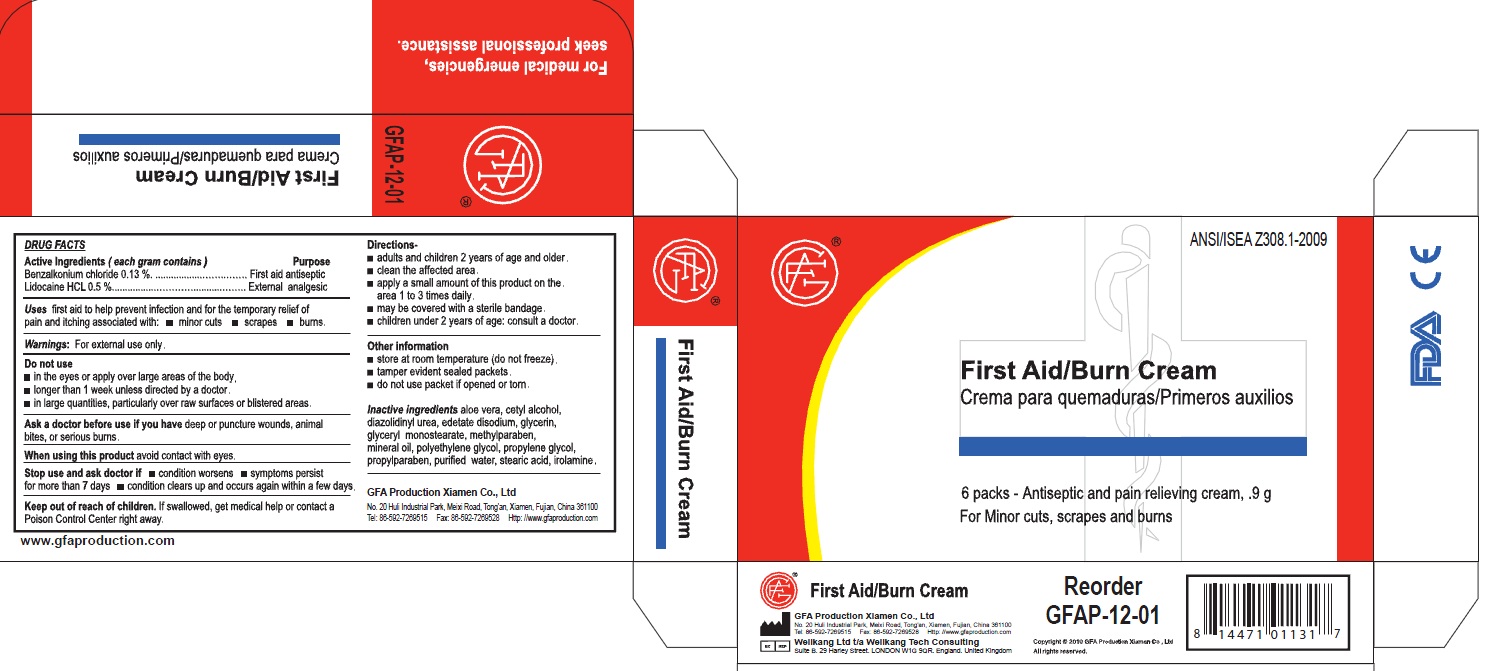
- PRINCIPAL DISPLAY PANEL
- Active Ingredients
- Purpose
- Uses
- Warnings
-
DO NOT USE
Do not use: In eyes, in large quantities, over raw blistered areas, or on deep puncture wounds, animal bites or serious burns, for more than one week
Do not use:
in the eyes or apply over large areas of the body.
longer than 1 week unless directed by a doctor.
in large quantities, particularly over raw surfaces or blistered areas.
Ask a doctor before use if you have deep puncture wounds, animal bites or serious burns.
When using this product, avoid contact with the eyes.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
- Inactive Ingredients
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
- Warnings
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- STORAGE AND HANDLING
- Inactive Ingredient
-
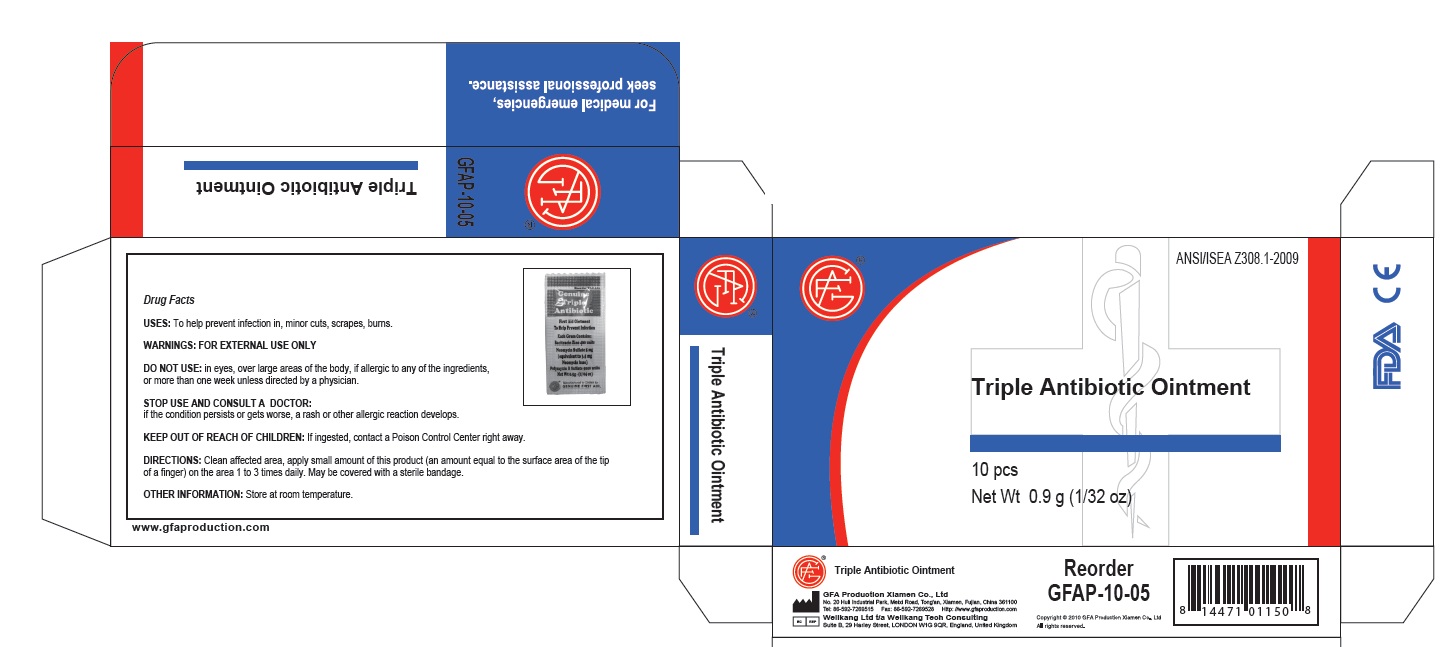
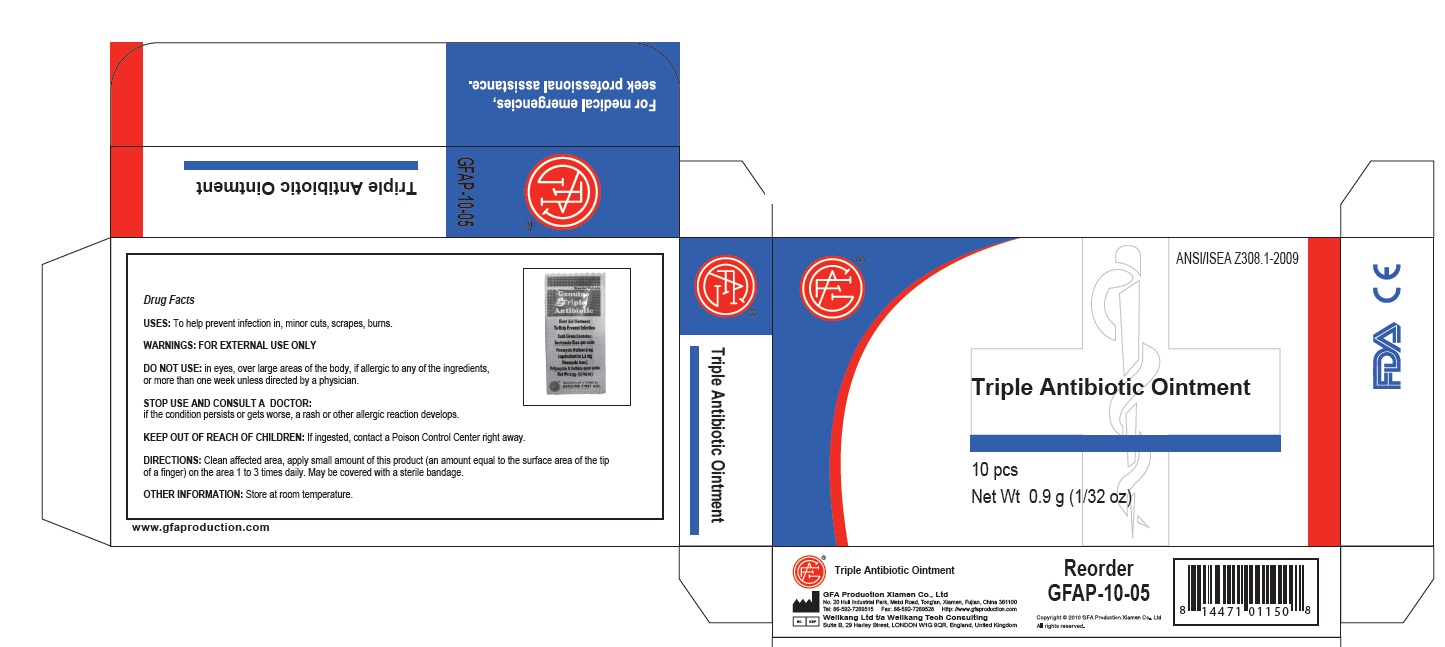
PRINCIPAL DISPLAY PANEL
Genuine Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID.
Triple Antibiotic Ointment 10pcs
Net wt. 0.9g (1/32oz)
100
Triple Antibiotic
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GFA FIRST AID
benzalkonium chloride, benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52124-1164 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-1164-3 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 PACKET 8.0 mL Part 2 6 PACKET 5.4 g Part 3 10 TUBE 5.0 g Part 1 of 3 ANTISEPTIC TOWELETTE
benzalkonium chloride swabProduct Information Item Code (Source) NDC:52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.4 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0001-2 1 in 1 BOX 1 0.8 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 04/07/2011 Part 2 of 3 GENUINE FIRST AID BURN ANTISEPTIC PAIN RELIEF WITH ALOE
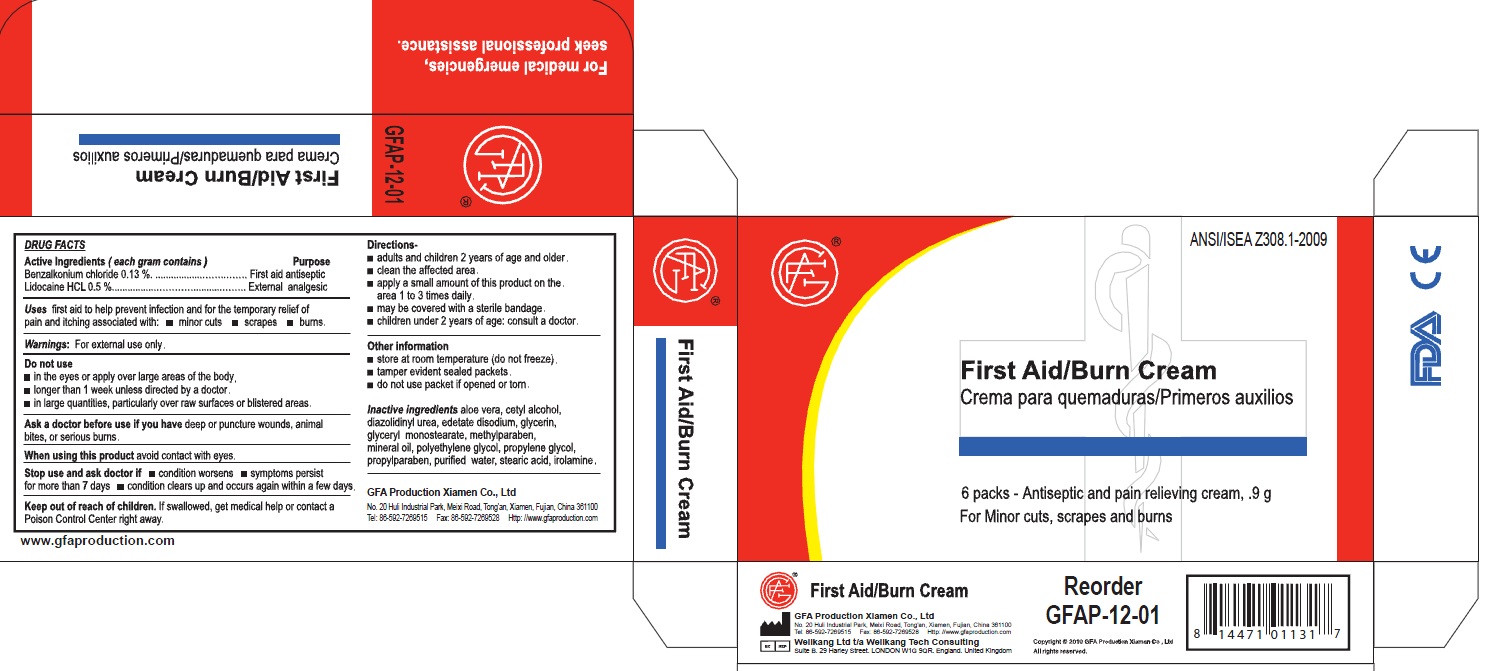
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC:52124-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CETYL ALCOHOL (UNII: 936JST6JCN) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0004-2 6 in 1 BOX 1 0.9 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333A 04/13/2011 Part 3 of 3 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0003-2 10 in 1 BOX 1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 04/13/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333E 04/13/2011 Labeler - Genuine First Aid, LLC (619609857)