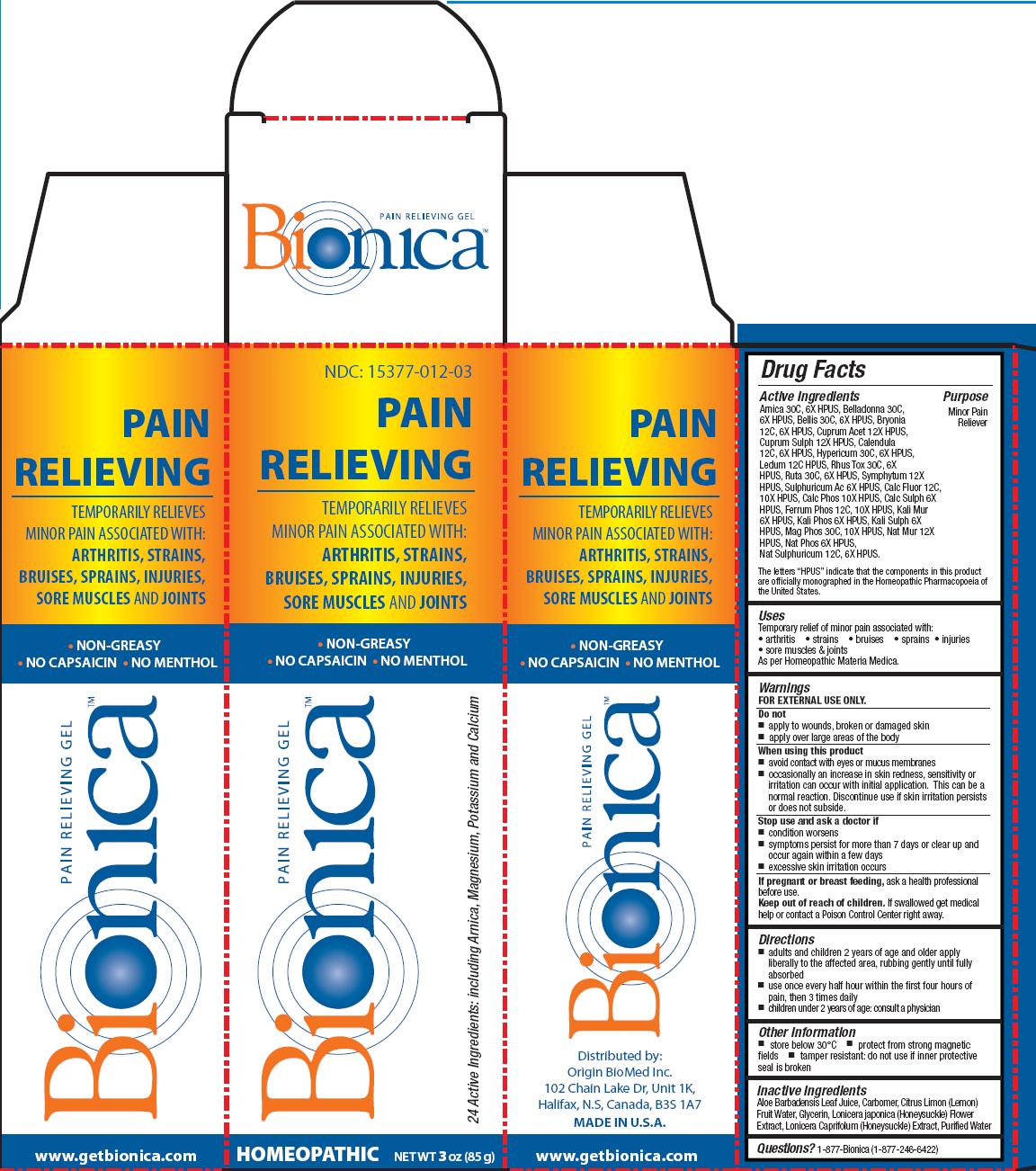
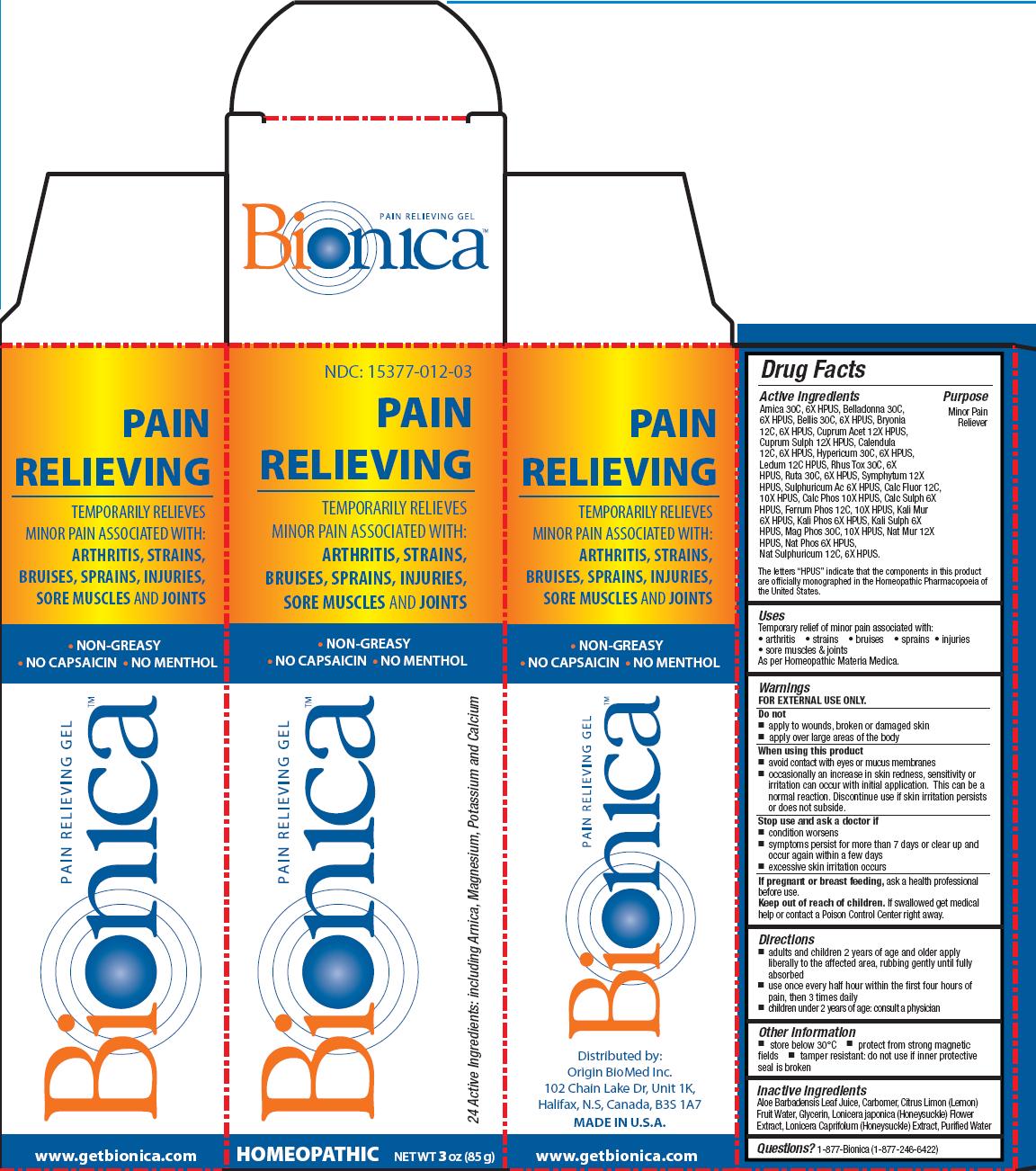
Label: BIONICA PAIN RELIEVING GEL- arnica, belladonna, bellis, bryonia, cuprum acet, cuprum sulph, calendula, hypericum, ledum, rhus tox, ruta, symphytum, sulphuricum ac, calc fluor, calc phos, calch sulph, ferrum phos, kali sulph, mag phos, nat mur, nat phos, nat sulphuricum gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 15377-012-03 - Packager: Origin BioMed Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 23, 2014
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
Arnica 30C, 6X HPUS, Belladonna 30C,6X HPUS, Bellis 30C, 6X HPUS, Bryonia 12C, 6X HPUS, Cuprum Acet 12X HPUS,Cuprum Sulph 12X HPUS, Calendula 12C, 6X HPUS, Hypericum 30C, 6X HPUS, Ledum 12C HPUS, Rhus Tox 30C, 6X HPUS, Ruta 30C, 6X HPUS, Symphytum 12X HPUS, Sulphuricum Ac 6X HPUS, Calc Fluor 12C,10X HPUS, Calc Phos 10X HPUS, Calc Sulph 6X HPUS, Ferrum Phos 12C, 10X HPUS, Kali Mur 6X HPUS, Kali Phos 6X HPUS, Kali Sulph 6X HPUS, Mag Phos 30C, 10X HPUS, Nat Mur 12X HPUS, Nat Phos 6X HPUS, Nat Sulphuricum 12C, 6X HPUS.
The letters “HPUS” indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States
- Purpose
- Uses
-
Warnings
FOR EXTERNAL USE ONLY.
When using this product
- avoid contact with eyes or mucus membranes
- occasionally an increase in skin redness, sensitivity or irritation can occur with initial application. This can be a normal reaction. Discontinue use if skin irritation persists or does not subside.
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- DESCRIPTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
BIONICA PAIN RELIEVING GEL
arnica, belladonna, bellis, bryonia, cuprum acet, cuprum sulph, calendula, hypericum, ledum, rhus tox, ruta, symphytum, sulphuricum ac, calc fluor, calc phos, calch sulph, ferrum phos, kali sulph, mag phos, nat mur, nat phos, nat sulphuricum gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15377-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) (ARNICA MONTANA FLOWER - UNII:OZ0E5Y15PZ) ARNICA MONTANA FLOWER 30 [hp_C] in 85 g ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 85 g BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 30 [hp_C] in 85 g BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 12 [hp_C] in 85 g CUPRIC ACETATE (UNII: 39M11XPH03) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC ACETATE 12 [hp_X] in 85 g CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 12 [hp_X] in 85 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 12 [hp_C] in 85 g HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 30 [hp_C] in 85 g LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 12 [hp_C] in 85 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 30 [hp_C] in 85 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 30 [hp_C] in 85 g COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 12 [hp_X] in 85 g SULFURIC ACID (UNII: O40UQP6WCF) (SULFURIC ACID - UNII:O40UQP6WCF) SULFURIC ACID 6 [hp_X] in 85 g CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 12 [hp_C] in 85 g TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 10 [hp_X] in 85 g CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 6 [hp_X] in 85 g FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_C] in 85 g POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 6 [hp_X] in 85 g POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 6 [hp_X] in 85 g POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 6 [hp_X] in 85 g MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 30 [hp_C] in 85 g SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 12 [hp_C] in 85 g SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] in 85 g SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE 6 [hp_X] in 85 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15377-012-03 1 in 1 CARTON 1 85 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/03/2013 Labeler - Origin BioMed Inc. (206071222) Establishment Name Address ID/FEI Business Operations ABCO Laboratories, Inc. 029618279 manufacture(15377-012) Establishment Name Address ID/FEI Business Operations C-Care LLC 120585950 manufacture(15377-012)