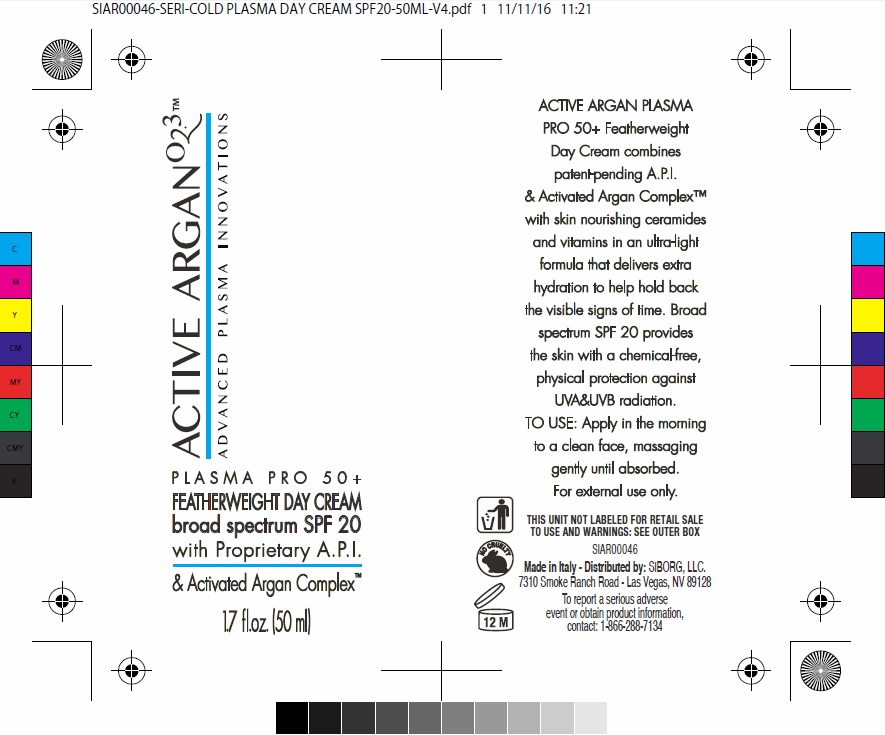
ACTIVE ARGAN 02.3(TM) PLASMA PRO 50 FEATHERWEIGHT DAY BROAD SPECTRUM SPF 20 WITH PROPRIETARY A.P.I. AND ACTIVATED ARGAN COMPLEX(TM)- zinc oxide cream
SIBORG
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE ARGAN 02.3(TM) PLASMA PRO 50+ FEATHERWEIGHT DAY CREAM BROAD SPECTRUM SPF 20 WITH PROPRIETARY A.P.I. & ACTIVATED ARGAN COMPLEX(TM)
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures ( See Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally and evenly 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
Inactive ingredients
water (aqua) (*oxygen-rich purified water), dicaprylyl carbonate, dimethicone, glycerin, caprylic/capric triglyceride, cetyl PEG/PPG-10/1 dimethicone, phenyl trimethicone, cetyl dimethicone, sodium chloride, hydrogenated dimer dilinoleyl/dimethylcarbonate copolymer, argania spinosa kernel oil (*specially-ozonized organic argan oil), tocopherol (*GMO-free vegetable vitamin E), wheat (triticum vulgare) germ oil, amyris balsamifera bark oil, tocopheryl acetate, ceramide 1, ceramide 6-II, ceramide 3, phytosphingosine, acacia decurrens/jojoba/sunflower seed wax/polyglyceryl-3 esters, triethoxycaprylylsilane, polyglyceryl-3 diisostearate, isopropyl myristate, BHT, carbomer, xanthan gum, sodium lauroyl lactylate, cholesterol, ethylhexylglycerin, fragrance (*100% natural fragrance), phenoxyethanol
| ACTIVE ARGAN 02.3(TM) PLASMA PRO 50 FEATHERWEIGHT DAY BROAD SPECTRUM SPF 20 WITH PROPRIETARY A.P.I. AND ACTIVATED ARGAN COMPLEX(TM)
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SIBORG (102875148) |