BACITRACIN ZINC- bacitracin zinc ointment
E. Fougera & Co. a division of Fougera Pharmaceuticals Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
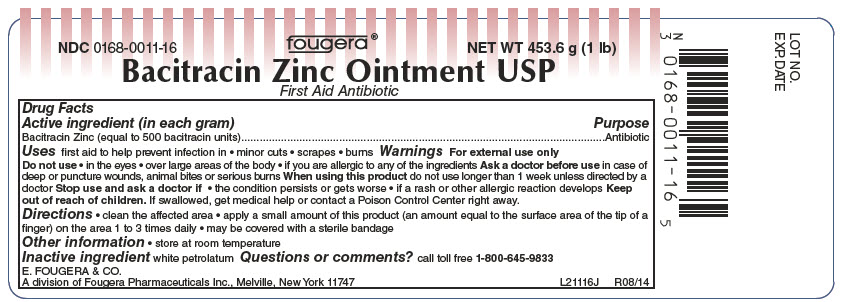
Bacitracin Zinc Ointment USP
Warnings: For external use only
Do not use
- •
- in the eyes
- •
- over large areas of the body
- •
- if you are allergic to any of the ingredients
Ask doctor before use in case of deep or puncture wounds, animal bites or serious burns
When using this product do not use longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- •
- the condition persists or gets worse
- •
- if a rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
- •
- clean the affected area
- •
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- •
- may be covered with a sterile bandage
Other information:
- •
- do not use if seal is punctured or is not visible. To open, reverse cap to puncture seal
- •
- store at room temperature
- •
- see crimp of tube for Lot Number and Exp. Date
Questions or comments? call toll free 1-800-645-9833
E. FOUGERA & CO.
A division of Fougera Pharmaceuticals Inc.
Melville, NY 11747
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 4 Oz CONTAINER
- NDC 0168-0011-04
Fougera®
NET WT 113.4 g (4 Oz)
FOR HOSPITAL USE ONLY
Bacitracin Zinc Ointment USP
First Aid Antibiotic
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 4 Oz Carton
Fougera ®
Bacitracin Zinc
Ointment USP
First Aid Antibiotic
- NDC 0168-0011-04
- FOR HOSPITAL USE ONLY
NET WT 113.4 g (4 Oz)
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 1/32 Oz CONTAINER
Fougera®
- First Aid Antibiotic
Bacitracin Zinc
Ointment USP
Uses: First aid to help prevent infection
in . minor cuts . scrapes . burns
NET WT. 0.9 g (1/32 Oz)
- NDC 0168-0111-09
| BACITRACIN ZINC
bacitracin zinc ointment |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| BACITRACIN ZINC
bacitracin zinc ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. (043838424) |