Label: AMITIZA- lubiprostone capsule, gelatin coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 35356-500-60 - Packager: Lake Erie Medical DBA Quality Care Products LLC
- This is a repackaged label.
- Source NDC Code(s): 64764-240
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 25, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AMITIZA safely and effectively. See full prescribing information for AMITIZA.
AMITIZA (lubiprostone) capsule, gelatin coated for oral use
Initial U.S. Approval: 2006RECENT MAJOR CHANGES
Dosage and Administration (2) 2/2011 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Chronic idiopathic constipation
- 24 mcg taken twice daily orally with food and water (2.1)
Reduce the dosage in patients with moderate and severe hepatic impairment (2.1)
Irritable bowel syndrome with constipation
- 8 mcg taken twice daily orally with food and water (2.2)
Reduce the dosage in patients with severe hepatic impairment (2.2)
DOSAGE FORMS AND STRENGTHS
- Gelatin capsules: 8 mcg and 24 mcg (3)
CONTRAINDICATIONS
- Patients with known or suspected mechanical gastrointestinal obstruction should not receive Amitiza (4)
WARNINGS AND PRECAUTIONS
- Women who could become pregnant should have a negative pregnancy test prior to beginning therapy and should be capable of complying with effective contraceptive measures (8.1)
- Use during pregnancy only if the potential benefit justifies the potential risk to the fetus (5.1)
- Patients may experience nausea; concomitant administration of food may reduce this symptom (5.2)
- Do not prescribe for patients that have severe diarrhea (5.3)
- Patients taking Amitiza may experience dyspnea within an hour of first dose. This symptom generally resolves within 3 hours, but may recur with repeat dosing (5.4)
- Evaluate patients with symptoms suggestive of mechanical gastrointestinal obstruction prior to initiating treatment with Amitiza (5.5)
ADVERSE REACTIONS
- Most common adverse reactions (incidence > 4%) in chronic idiopathic constipation are nausea, diarrhea, headache, abdominal pain, abdominal distension, and flatulence (6.1)
- Most common adverse reactions (incidence > 4%) in irritable bowel syndrome with constipation are nausea, diarrhea, and abdominal pain (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-877-825-3327 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2011
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Chronic Idiopathic Constipation
1.2 Irritable Bowel Syndrome with Constipation
2 DOSAGE AND ADMINISTRATION
2.1 Chronic Idiopathic Constipation
2.2 Irritable Bowel Syndrome with Constipation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pregnancy
5.2 Nausea
5.3 Diarrhea
5.4 Dyspnea
5.5 Bowel Obstruction
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chronic Idiopathic Constipation
14.2 Irritable Bowel Syndrome with Constipation
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Dosing Instructions
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Amitiza should be taken orally with food and water. Physicians and patients should periodically assess the need for continued therapy.
2.1 Chronic Idiopathic Constipation
The recommended dose is 24 mcg twice daily orally with food and water.
Reduced dosage in patients with hepatic impairment
For patients with moderately impaired hepatic function (Child-Pugh Class B), the recommended dose is 16 mcg twice daily. For patients with severely impaired hepatic function (Child-Pugh Class C), the recommended dose is 8 mcg twice daily. If this dose is tolerated and an adequate response has not been obtained after an appropriate interval, doses can then be escalated to full dosing with appropriate monitoring of patient response.
2.2 Irritable Bowel Syndrome with Constipation
The recommended dose is 8 mcg twice daily orally with food and water.
Reduced dosage in patients with severe hepatic impairment
For patients with severely impaired hepatic function (Child-Pugh Class C), the recommended dose is 8 mcg once daily. If this dose is tolerated and an adequate response has not been obtained after an appropriate interval, doses can then be escalated to full dosing with appropriate monitoring of patient response. Dosage adjustment is not required for patients with moderately impaired hepatic function (Child-Pugh Class B).
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Pregnancy
The safety of Amitiza in pregnancy has not been evaluated in humans. In guinea pigs, lubiprostone has been shown to have the potential to cause fetal loss. Amitiza should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women who could become pregnant should have a negative pregnancy test prior to beginning therapy with Amitiza and should be capable of complying with effective contraceptive measures [see Use in Specific Populations (8.1)].
5.2 Nausea
Patients taking Amitiza may experience nausea. If this occurs, concomitant administration of food with Amitiza may reduce symptoms of nausea [see Adverse Reactions (6.1)].
5.3 Diarrhea
Amitiza should not be prescribed to patients that have severe diarrhea. Patients should be aware of the possible occurrence of diarrhea during treatment. Patients should be instructed to inform their physician if severe diarrhea occurs [see Adverse Reactions (6.1)].
5.4 Dyspnea
In clinical trials conducted to study Amitiza in treatment of chronic idiopathic constipation and IBS-C there were reports of dyspnea. This was reported at 2.5% of the treated chronic idiopathic constipation population and at 0.4% in the treated IBS-C population. Although not classified as serious adverse events, some patients discontinued treatment on study because of this event. There have been postmarketing reports of dyspnea when using Amitiza 24 mcg. Most have not been characterized as serious adverse events, but some patients have discontinued therapy because of dyspnea. These events have usually been described as a sensation of chest tightness and difficulty taking in a breath, and generally have an acute onset within 30–60 minutes after taking the first dose. They generally resolve within a few hours after taking the dose, but recurrence has been frequently reported with subsequent doses.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Chronic Idiopathic Constipation
Adverse reactions in dose-finding, efficacy, and long-term clinical studies: The data described below reflect exposure to Amitiza in 1175 patients with chronic idiopathic constipation (29 at 24 mcg once daily, 1113 at 24 mcg twice daily, and 33 at 24 mcg three times daily) over 3- or 4-week, 6-month, and 12-month treatment periods; and from 316 patients receiving placebo over short-term exposure (≤ 4 weeks). The total population (N = 1491) had a mean age of 49.7 (range 19–86) years; was 87.1% female; 84.8% Caucasian, 8.5% African American, 5.0% Hispanic, 0.9% Asian; and 15.5% elderly (≥ 65 years of age). Table 1 presents data for the adverse reactions that occurred in at least 1% of patients who received Amitiza 24 mcg twice daily and that occurred more frequently with study drug than placebo. In addition, corresponding adverse reaction incidences in patients receiving Amitiza 24 mcg once daily is shown.
Table 1: Percent of Patients with Adverse Reactions (Chronic Idiopathic Constipation) 1Includes only those events associated with treatment (possibly, probably, or definitely related, as assessed by the investigator).
2This term combines "abdominal tenderness," "abdominal rigidity," "gastrointestinal discomfort," and "abdominal discomfort."
System/Adverse Reaction1Placebo
N = 316
%Amitiza
24 mcg
Once Daily
N = 29
%Amitiza
24 mcg
Twice Daily
N = 1113
%Gastrointestinal disorders Nausea
3 17 29 Diarrhea
< 1 7 12 Abdominal pain
3 3 8 Abdominal distension
2 - 6 Flatulence
2 3 6 Vomiting
- - 3 Loose stools
- - 3 Abdominal discomfort2
< 1 3 2 Dyspepsia
< 1 - 2 Dry mouth
< 1 - 1 Stomach discomfort
< 1 - 1 Nervous system disorders Headache
5 3 11 Dizziness
< 1 3 3 General disorders and site administration conditions Edema
< 1 - 3 Fatigue
< 1 - 2 Chest discomfort/pain
- 3 2 Respiratory, thoracic, and mediastinal disorders Dyspnea
- 3 2 Nausea: Approximately 29% of patients who received Amitiza 24 mcg twice daily experienced an adverse reaction of nausea; 4% of patients had severe nausea while 9% of patients discontinued treatment due to nausea. The rate of nausea associated with Amitiza (any dosage) was substantially lower among male (7%) and elderly patients (18%). Further analysis of the safety data revealed that long-term exposure to Amitiza does not appear to place patients at an elevated risk for experiencing nausea. The incidence of nausea increased in a dose-dependent manner with the lowest overall incidence for nausea reported at the 24 mcg once daily dosage (17%). In open-labeled, long-term studies, patients were allowed to adjust the dosage of Amitiza down to 24 mcg once daily from 24 mcg twice daily if experiencing nausea. Nausea decreased when Amitiza was administered with food. No patients in the clinical studies were hospitalized due to nausea.
Diarrhea: Approximately 12% of patients who received Amitiza 24 mcg twice daily experienced an adverse reaction of diarrhea; 2% of patients had severe diarrhea while 2% of patients discontinued treatment due to diarrhea.
Electrolytes: No serious adverse reactions of electrolyte imbalance were reported in clinical studies, and no clinically significant changes were seen in serum electrolyte levels in patients receiving Amitiza.
Less common adverse reactions: The following adverse reactions (assessed by investigator as probably or definitely related to treatment) occurred in less than 1% of patients receiving Amitiza 24 mcg twice daily in clinical studies, occurred in at least two patients, and occurred more frequently in patients receiving study drug than those receiving placebo: fecal incontinence, muscle cramp, defecation urgency, frequent bowel movements, hyperhidrosis, pharyngolaryngeal pain, intestinal functional disorder, anxiety, cold sweat, constipation, cough, dysgeusia, eructation, influenza, joint swelling, myalgia, pain, syncope, tremor, decreased appetite.
Irritable Bowel Syndrome with Constipation
Adverse reactions in dose-finding, efficacy, and long-term clinical studies: The data described below reflect exposure to Amitiza 8 mcg twice daily in 1011 patients with IBS-C for up to 12 months and from 435 patients receiving placebo twice daily for up to 16 weeks. The total population (N = 1267) had a mean age of 46.5 (range 18–85) years; was 91.6% female; 77.5% Caucasian, 12.9% African American, 8.6% Hispanic, 0.4% Asian; and 8.0% elderly (≥ 65 years of age). Table 2 presents data for the adverse reactions that occurred in at least 1% of patients who received Amitiza 8 mcg twice daily and that occurred more frequently with study drug than placebo.
Table 2: Percent of Patients with Adverse Reactions (IBS-C Studies) 1Includes only those events associated with treatment (possibly or probably related, as assessed by the investigator).
System/Adverse Reaction1Placebo
N = 435
%Amitiza
8 mcg
Twice Daily
N = 1011
%Gastrointestinal disorders Nausea
4 8 Diarrhea
4 7 Abdominal pain
5 5 Abdominal distension
2 3 Less common adverse reactions: The following adverse reactions (assessed by investigator as probably related to treatment) occurred in less than 1% of patients receiving Amitiza 8 mcg twice daily in clinical studies, occurred in at least two patients, and occurred more frequently in patients receiving study drug than those receiving placebo: dyspepsia, loose stools, vomiting, fatigue, dry mouth, edema, increased alanine aminotransferase, increased aspartate aminotransferase, constipation, eructation, gastroesophageal reflux disease, dyspnea, erythema, gastritis, increased weight, palpitations, urinary tract infection, anorexia, anxiety, depression, fecal incontinence, fibromyalgia, hard feces, lethargy, rectal hemorrhage, pollakiuria.
One open-labeled, long-term clinical study was conducted in patients with IBS-C receiving Amitiza 8 mcg twice daily. This study comprised 476 intent-to-treat patients (mean age 47.5 [range 21–82] years; 93.5% female; 79.2% Caucasian, 11.6% African American, 8.6% Hispanic, 0.2% Asian; 7.8% ≥ 65 years of age) who were treated for an additional 36 weeks following an initial 12–16-week, double-blinded treatment period. The adverse reactions that were reported during this study were similar to those observed in the two double-blinded, controlled studies.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of Amitiza 24 mcg for the treatment of chronic idiopathic constipation. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Voluntary reports of adverse reactions occurring with the use of Amitiza include the following: syncope, allergic-type reactions (including rash, swelling, and throat tightness), malaise, increased heart rate, muscle cramps or muscle spasms, and asthenia.
-
7 DRUG INTERACTIONS
Based upon the results of in vitro human microsome studies, there is low likelihood of drug–drug interactions. In vitro studies using human liver microsomes indicate that cytochrome P450 isoenzymes are not involved in the metabolism of lubiprostone. Further in vitro studies indicate microsomal carbonyl reductase may be involved in the extensive biotransformation of lubiprostone to the metabolite M3 [see Clinical Pharmacology (12.3)]. Additionally, in vitro studies in human liver microsomes demonstrate that lubiprostone does not inhibit cytochrome P450 isoforms 3A4, 2D6, 1A2, 2A6, 2B6, 2C9, 2C19, or 2E1, and in vitro studies of primary cultures of human hepatocytes show no induction of cytochrome P450 isoforms 1A2, 2B6, 2C9, and 3A4 by lubiprostone. No drug–drug interaction studies have been performed. Based on the available information, no protein binding–mediated drug interactions of clinical significance are anticipated.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: Pregnancy Category C. [See Warnings and Precautions(5.1).]
Teratology studies with lubiprostone have been conducted in rats at oral doses up to 2000 mcg/kg/day (approximately 332 times the recommended human dose, based on body surface area), and in rabbits at oral doses of up to 100 mcg/kg/day (approximately 33 times the recommended human dose, based on body surface area). Lubiprostone was not teratogenic in rats or rabbits. In guinea pigs, lubiprostone caused fetal loss at repeated doses of 10 and 25 mcg/kg/day (approximately 2 and 6 times the highest recommended human dose, respectively, based on body surface area) administered on days 40 to 53 of gestation.
There are no adequate and well-controlled studies in pregnant women. However, during clinical testing of Amitiza, six women became pregnant. Per protocol, Amitiza was discontinued upon pregnancy detection. Four of the six women delivered healthy babies. The fifth woman was monitored for 1 month following discontinuation of study drug, at which time the pregnancy was progressing as expected; the patient was subsequently lost to follow-up. The sixth pregnancy was electively terminated.
Amitiza should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. If a woman is or becomes pregnant while taking the drug, the patient should be apprised of the potential hazard to the fetus.
8.3 Nursing Mothers
It is not known whether lubiprostone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from lubiprostone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
Chronic Idiopathic Constipation
The efficacy of Amitiza in the elderly (≥ 65 years of age) subpopulation was consistent with the efficacy in the overall study population. Of the total number of constipated patients treated in the dose-finding, efficacy, and long-term studies of Amitiza, 15.5% were ≥ 65 years of age, and 4.2% were ≥ 75 years of age. Elderly patients taking Amitiza (any dosage) experienced a lower incidence rate of associated nausea compared to the overall study population taking Amitiza (18% vs. 29%, respectively).
Irritable Bowel Syndrome with Constipation
The safety profile of Amitiza in the elderly (≥ 65 years of age) subpopulation (8.0% were ≥ 65 years of age and 1.8% were ≥ 75 years of age) was consistent with the safety profile in the overall study population. Clinical studies of Amitiza did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients.
8.6 Renal Impairment
No dosage adjustment is required in patients with renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh Class B) and severe hepatic impairment (Child-Pugh Class C) experienced markedly higher systemic drug exposure; therefore, dosing with Amitiza should be modified in these patients [see Dosage and Administration (2.1, 2.2) and Clinical Pharmacology (12.3)]. No dosage adjustment is required in patients with mild hepatic impairment (Child-Pugh Class A).
-
10 OVERDOSAGE
There have been two confirmed reports of overdosage with Amitiza. The first report involved a 3-year-old child who accidentally ingested 7 or 8 capsules of 24 mcg of Amitiza and fully recovered. The second report was a study patient who self-administered a total of 96 mcg of Amitiza per day for 8 days. The patient experienced no adverse reactions during this time. Additionally, in a Phase 1 cardiac repolarization study, 38 of 51 healthy volunteers given a single oral dose of 144 mcg of Amitiza (6 times the highest recommended dose) experienced an adverse event that was at least possibly related to the study drug. Adverse reactions that occurred in at least 1% of these volunteers included the following: nausea (45%), diarrhea (35%), vomiting (27%), dizziness (14%), headache (12%), abdominal pain (8%), flushing/hot flash (8%), retching (8%), dyspnea (4%), pallor (4%), stomach discomfort (4%), anorexia (2%), asthenia (2%), chest discomfort (2%), dry mouth (2%), hyperhidrosis (2%), and syncope (2%).
-
11 DESCRIPTION
Amitiza (lubiprostone) is chemically designated as (–)-7-[(2R,4aR,5R,7aR)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[b]pyran-5-yl]heptanoic acid. The molecular formula of lubiprostone is C20H32F2O5 with a molecular weight of 390.46 and a chemical structure as follows:
Lubiprostone drug substance occurs as white, odorless crystals or crystalline powder, is very soluble in ether and ethanol, and is practically insoluble in hexane and water. Amitiza is available as an imprinted, oval, soft gelatin capsule in two strengths. Pink capsules contain 8 mcg of lubiprostone and the following inactive ingredients: medium-chain triglycerides, gelatin, sorbitol, ferric oxide, titanium dioxide, and purified water. Orange capsules contain 24 mcg of lubiprostone and the following inactive ingredients: medium-chain triglycerides, gelatin, sorbitol, FD&C Red #40, D&C Yellow #10, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lubiprostone is a locally acting chloride channel activator that enhances a chloride-rich intestinal fluid secretion without altering sodium and potassium concentrations in the serum. Lubiprostone acts by specifically activating ClC-2, which is a normal constituent of the apical membrane of the human intestine, in a protein kinase A–independent fashion. By increasing intestinal fluid secretion, lubiprostone increases motility in the intestine, thereby facilitating the passage of stool and alleviating symptoms associated with chronic idiopathic constipation. Patch clamp cell studies in human cell lines have indicated that the majority of the beneficial biological activity of lubiprostone and its metabolites is observed only on the apical (luminal) portion of the gastrointestinal epithelium. Additionally, activation of ClC-2 by lubiprostone has been shown to stimulate recovery of mucosal barrier function via the restoration of tight junction protein complexes in ex vivo studies of ischemic porcine intestine.
12.2 Pharmacodynamics
Although the pharmacologic effects of lubiprostone in humans have not been fully evaluated, animal studies have shown that oral administration of lubiprostone increases chloride ion transport into the intestinal lumen, enhances fluid secretion into the bowels, and improves fecal transit.
12.3 Pharmacokinetics
Lubiprostone has low systemic availability following oral administration and concentrations of lubiprostone in plasma are below the level of quantitation (10 pg/mL). Therefore, standard pharmacokinetic parameters such as area under the curve (AUC), maximum concentration (Cmax), and half-life (t½) cannot be reliably calculated. However, the pharmacokinetic parameters of M3 (only measurable active metabolite of lubiprostone) have been characterized. Gender has no effect on the pharmacokinetics of M3 following the oral administration of lubiprostone.
Absorption
Concentrations of lubiprostone in plasma are below the level of quantitation (10 pg/mL) because lubiprostone has a low systemic availability following oral administration. Peak plasma levels of M3, after a single oral dose with 24 mcg of lubiprostone, occurred at approximately 1.10 hours. The Cmax was 41.5 pg/mL and the mean AUC0–t was 57.1 pg∙hr/mL. The AUC0–t of M3 increases dose proportionally after single 24-mcg and 144-mcg doses of lubiprostone.
Distribution
In vitro protein binding studies indicate lubiprostone is approximately 94% bound to human plasma proteins. Studies in rats given radiolabeled lubiprostone indicate minimal distribution beyond the gastrointestinal tissues. Concentrations of radiolabeled lubiprostone at 48 hours post-administration were minimal in all tissues of the rats.
Metabolism
The results of both human and animal studies indicate that lubiprostone is rapidly and extensively metabolized by 15-position reduction, α-chain β-oxidation, and ω-chain ω-oxidation. These biotransformations are not mediated by the hepatic cytochrome P450 system but rather appear to be mediated by the ubiquitously expressed carbonyl reductase. M3, a metabolite of lubiprostone found in both humans and animals, is formed by the reduction of the carbonyl group at the 15-hydroxy moiety that consists of both α-hydroxy and β-hydroxy epimers. M3 makes up less than 10% of the dose of radiolabeled lubiprostone. Animal studies have shown that metabolism of lubiprostone rapidly occurs within the stomach and jejunum, most likely in the absence of any systemic absorption. This is presumed to be the case in humans as well.
Elimination
Lubiprostone could not be detected in plasma; however, M3 has a t½ ranging from 0.9 to 1.4 hours. After a single oral dose of 72 mcg of 3H-labeled lubiprostone, 60% of total administered radioactivity was recovered in the urine within 24 hours and 30% of total administered radioactivity was recovered in the feces by 168 hours. Lubiprostone and M3 are only detected in trace amounts in human feces.
Food Effect
A study was conducted with a single 72-mcg dose of 3H-labeled lubiprostone to evaluate the potential of a food effect on lubiprostone absorption, metabolism, and excretion. Pharmacokinetic parameters of total radioactivity demonstrated that Cmax decreased by 55% while AUC0–∞ was unchanged when lubiprostone was administered with a high-fat meal. The clinical relevance of the effect of food on the pharmacokinetics of lubiprostone is not clear. However, lubiprostone was administered with food and water in a majority of clinical trials.
Special Populations
Renal Impairment
Sixteen subjects, 34–47 years old (8 severe renally impaired subjects [creatinine clearance (CrCl) < 20 mL/min] who required hemodialysis and 8 control subjects with normal renal function [CrCl > 80 mL/min]), received a single oral 24-mcg dose of Amitiza. Following administration, lubiprostone plasma concentrations were below the limit of quantitation (10 pg/mL). Plasma concentrations of M3 were within the range of exposure from previous clinical experience with Amitiza. Thus there is no need for Amitiza dosage adjustment in patients with impaired renal function.
Hepatic Impairment
Twenty-five subjects, 38–78 years old (9 with severe hepatic impairment [Child-Pugh Class C], 8 with moderate impairment [Child-Pugh Class B], and 8 with normal liver function), received either 12 mcg or 24 mcg of Amitiza under fasting conditions. Following administration, lubiprostone plasma concentrations were below the limit of quantitation (10 pg/mL) except for two subjects. In moderately and severely impaired subjects, the Cmax and AUC0–t of M3 were increased, as shown in Table 3.
Table 3: Pharmacokinetic Parameters of M3 for Subjects with Normal or Impaired Liver Function following Dosing with Amitiza Liver Function Status Mean (SD) AUC0–t
(pg•hr/mL)% Change vs. Normal Mean (SD) Cmax
(pg/mL)% Change vs. Normal Normal (n=8) 39.6 (18.7) n.a. 37.5 (15.9) n.a. Child-Pugh Class B (n=8) 119 (104) +119 70.9 (43.5) +66 Child-Pugh Class C (n=8) 234 (61.6) +521 114 (59.4) +183 These results demonstrate that there is a correlation between increased exposure of M3 and severity of hepatic impairment. In conjunction with the clinical safety results, which demonstrate an increased incidence and severity of adverse events in subjects with greater severity of hepatic impairment, the starting dosage should be reduced in patients with hepatic impairment receiving Amitiza [see Dosage and Administration (2.1, 2.2)]. No dosing adjustment is required in patients with mild hepatic impairment (Child-Pugh Class A).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two 2-year oral (gavage) carcinogenicity studies (one in Crl:B6C3F1 mice and one in Sprague-Dawley rats) were conducted with lubiprostone. In the 2-year carcinogenicity study in mice, lubiprostone doses of 25, 75, 200, and 500 mcg/kg/day (approximately 2, 6, 17, and 42 times the highest recommended human dose, respectively, based on body surface area) were used. In the 2-year rat carcinogenicity study, lubiprostone doses of 20, 100, and 400 mcg/kg/day (approximately 3, 17, and 68 times the highest recommended human dose, respectively, based on body surface area) were used. In the mouse carcinogenicity study, there was no significant increase in any tumor incidences. There was a significant increase in the incidence of interstitial cell adenoma of the testes in male rats at the 400 mcg/kg/day dose. In female rats, treatment with lubiprostone produced hepatocellular adenoma at the 400 mcg/kg/day dose.
Mutagenesis
Lubiprostone was not genotoxic in the in vitro Ames reverse mutation assay, the in vitro mouse lymphoma (L5178Y TK +/–) forward mutation assay, the in vitro Chinese hamster lung (CHL/IU) chromosomal aberration assay, and the in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Lubiprostone, at oral doses of up to 1000 mcg/kg/day, had no effect on the fertility and reproductive function of male and female rats. However, the number of implantation sites and live embryos were significantly reduced in rats at the 1000 mcg/kg/day dose as compared to control. The number of dead or resorbed embryos in the 1000 mcg/kg/day group was higher compared to the control group, but was not statistically significant. The 1000 mcg/kg/day dose in rats is approximately 166 times the highest recommended human dose of 48 mcg/day, based on body surface area.
-
14 CLINICAL STUDIES
14.1 Chronic Idiopathic Constipation
Dose-finding Study
A dose-finding, double-blinded, parallel-group, placebo-controlled, Phase 2 study was conducted in patients with chronic idiopathic constipation. Following a 2-week baseline/washout period, patients (N = 127) were randomized to receive placebo (n = 33), Amitiza 24 mcg/day (24 mcg once daily; n = 29), Amitiza 48 mcg/day (24 mcg twice daily; n = 32), or Amitiza 72 mcg/day (24 mcg three times daily; n = 33) for 3 weeks. Patients were chosen for participation based on their need for relief of constipation, which was defined as less than 3 spontaneous bowel movements (SBMs) per week. The primary efficacy variable was the daily average number of SBMs.
The study demonstrated that all patients who took Amitiza experienced a noticeable improvement in clinical response. Based on the efficacy analysis, there was no statistically significant improvement in the clinical response beyond a total daily dose of 24 mcg during treatment weeks 2 and 3 (Figure 1).
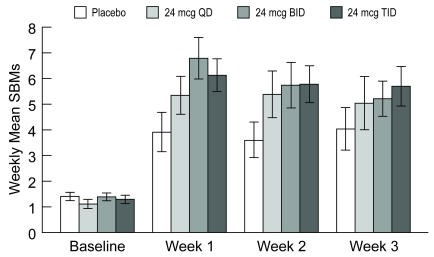
Figure 1: Weekly Mean (± Standard Error) Spontaneous Bowel Movements (Dose-finding Study)
Efficacy Studies
Two double-blinded, placebo-controlled studies of identical design were conducted in patients with chronic idiopathic constipation. Chronic idiopathic constipation was defined as, on average, less than 3 SBMs per week along with one or more of the following symptoms of constipation for at least 6 months prior to randomization: 1) very hard stools for at least a quarter of all bowel movements; 2) sensation of incomplete evacuation following at least a quarter of all bowel movements; and 3) straining with defecation at least a quarter of the time.
Following a 2-week baseline/washout period, a total of 479 patients (mean age 47.2 [range 20–81] years; 88.9% female; 80.8% Caucasian, 9.6% African American, 7.3% Hispanic, 1.5% Asian; 10.9% ≥ 65 years of age) were randomized and received Amitiza 24 mcg twice daily (48 mcg/day) or placebo twice daily for 4 weeks. The primary endpoint of the studies was SBM frequency. The studies demonstrated that patients treated with Amitiza had a higher frequency of SBMs during Week 1 than the placebo patients. In both studies, results similar to those in Week 1 were also observed in Weeks 2, 3, and 4 of therapy (Table 4).
Table 4: Spontaneous Bowel Movement Frequency Rates1 (Efficacy Studies) 1Frequency rates are calculated as 7 times (number of SBMs) / (number of days observed for that week).
Trial
Study Arm
Baseline
Mean ± SD
Median
Week 1
Mean ± SD
Median
Week 2
Mean ± SD
Median
Week 3
Mean ± SD
Median
Week 4
Mean ± SD
MedianWeek 1 Change
from Baseline
Mean ± SD
MedianWeek 4 Change
from Baseline
Mean ± SD
MedianPlacebo 1.6 ± 1.3
1.53.5 ± 2.3
3.03.2 ± 2.5
3.02.8 ± 2.2
2.02.9 ± 2.4
2.31.9 ± 2.2
1.51.3 ± 2.5
1.0Study 1 Amitiza
24 mcg
Twice Daily1.4 ± 0.8
1.55.7 ± 4.4
5.05.1 ± 4.1
4.05.3 ± 4.9
5.05.3 ± 4.7
4.04.3 ± 4.3
3.53.9 ± 4.6
3.0Placebo 1.5 ± 0.8
1.54.0 ± 2.7
3.53.6 ± 2.7
3.03.4 ± 2.8
3.03.5 ± 2.9
3.02.5 ± 2.6
1.51.9 ± 2.7
1.5Study 2 Amitiza
24 mcg
Twice Daily1.3 ± 0.9
1.55.9 ± 4.0
5.05.0 ± 4.2
4.05.6 ± 4.6
5.05.4 ± 4.8
4.34.6 ± 4.1
3.84.1 ± 4.8
3.0In both studies, Amitiza demonstrated increases in the percentage of patients who experienced SBMs within the first 24 hours after administration when compared to placebo (56.7% vs. 36.9% in Study 1 and 62.9% vs. 31.9% in Study 2, respectively). Similarly, the time to first SBM was shorter for patients receiving Amitiza than for those receiving placebo.
Signs and symptoms related to constipation, including abdominal bloating, abdominal discomfort, stool consistency, and straining, as well as constipation severity ratings, were also improved with Amitiza versus placebo. The results were consistent in subpopulation analyses for gender, race, and elderly patients (≥ 65 years of age).
Following 4 weeks of treatment with Amitiza 24 mcg twice daily, withdrawal of Amitiza did not result in a rebound effect.
Long-term Studies
Three open-labeled, long-term clinical safety and efficacy studies were conducted in patients with chronic idiopathic constipation receiving Amitiza 24 mcg twice daily. These studies comprised 871 patients (mean age 51.0 [range 19–86] years; 86.1% female; 86.9% Caucasian, 7.3% African American, 4.5% Hispanic, 0.7% Asian; 18.4% ≥ 65 years of age) who were treated for 6–12 months (24–48 weeks). Patients provided regular assessments of abdominal bloating, abdominal discomfort, and constipation severity. These studies demonstrated that Amitiza decreased abdominal bloating, abdominal discomfort, and constipation severity over the 6–12-month treatment periods.
14.2 Irritable Bowel Syndrome with Constipation
Efficacy Studies
Two double-blinded, placebo-controlled studies of similar design were conducted in patients with IBS-C. IBS was defined as abdominal pain or discomfort occurring over at least 6 months with two or more of the following: 1) relieved with defecation; 2) onset associated with a change in stool frequency; and 3) onset associated with a change in stool form. Patients were sub-typed as having IBS-C if they also experienced two of three of the following: 1) < 3 spontaneous bowel movements per week, 2) > 25% hard stools, and 3) > 25% spontaneous bowel movements associated with straining.
Following a 4-week baseline/washout period, a total of 1154 patients (mean age 46.6 [range 18–85] years; 91.6% female; 77.4% Caucasian, 13.2% African American, 8.5% Hispanic, 0.4% Asian; 8.3% ≥ 65 years of age) were randomized and received Amitiza 8 mcg twice daily (16 mcg/day) or placebo twice daily for 12 weeks. The primary efficacy endpoint was assessed weekly utilizing the patient's response to a global symptom relief question based on a 7-point, balanced scale ("significantly worse" to "significantly relieved"): "How would you rate your relief of IBS symptoms (abdominal discomfort/pain, bowel habits, and other IBS symptoms) over the past week compared to how you felt before you entered the study?"
The primary efficacy analysis was a comparison of the proportion of "overall responders" in each arm. A patient was considered an "overall responder" if the criteria for being designated a "monthly responder" were met in at least 2 of the 3 months on study. A "monthly responder" was defined as a patient who had reported "significantly relieved" for at least 2 weeks of the month or at least "moderately relieved" in all 4 weeks of that month. During each monthly evaluation period, patients reporting "moderately worse" or "significantly worse" relief, an increase in rescue medication use, or those who discontinued due to lack of efficacy, were deemed non-responders.
The percentage of patients in Study 1 qualifying as an "overall responder" was 13.8% in the group receiving Amitiza 8 mcg twice daily compared to 7.8% of patients receiving placebo twice daily. In Study 2, 12.1% of patients in the Amitiza 8 mcg group were "overall responders" versus 5.7% of patients in the placebo group. In both studies, the treatment differences between the placebo and Amitiza groups were statistically significant.
Results in men: The two randomized, placebo-controlled, double-blinded studies comprised 97 (8.4%) male patients, which is insufficient to determine whether men with IBS-C respond differently to Amitiza from women.
Study 1 also assessed the rebound effect from the withdrawal of Amitiza. Following 12 weeks of treatment with Amitiza 8 mcg twice daily, withdrawal of Amitiza did not result in a rebound effect.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Amitiza is available as an oval, soft gelatin capsule containing 8 mcg or 24 mcg of lubiprostone with "SPI" printed on one side. Amitiza is available as follows:
8-mcg pink capsule
- Bottles of 60 (NDC 64764-080-60)
24-mcg orange capsule
- Bottles of 60 (NDC 64764-240-60)
- Bottles of 100 (NDC 64764-240-10)
-
17 PATIENT COUNSELING INFORMATION
17.1 Dosing Instructions
Amitiza should be taken twice daily with food and water to reduce potential symptoms of nausea. The capsule should be taken once in the morning and once in the evening daily as prescribed. The capsule should be swallowed whole and should not be broken apart or chewed. Physicians and patients should periodically assess the need for continued therapy.
Patients on treatment who experience severe nausea, diarrhea, or dyspnea should inform their physician. Patients taking Amitiza may experience dyspnea within an hour of the first dose. This symptom generally resolves within 3 hours, but may recur with repeat dosing.
Chronic Idiopathic Constipation
Patients should take a single 24 mcg capsule of Amitiza twice daily with food and water.
Irritable Bowel Syndrome with Constipation
Patients should take a single 8 mcg capsule of Amitiza twice daily with food and water.
Marketed by:
Sucampo Pharma Americas, Inc.
Bethesda, MD 20814and
Takeda Pharmaceuticals America, Inc.
Deerfield, IL 60015Amitiza® is a registered trademark of Sucampo Pharmaceuticals, Inc.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMITIZA
lubiprostone capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:35356-500(NDC:64764-240) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lubiprostone (UNII: 7662KG2R6K) (lubiprostone - UNII:7662KG2R6K) lubiprostone 24 ug Inactive Ingredients Ingredient Name Strength medium-chain triglycerides (UNII: C9H2L21V7U) gelatin (UNII: 2G86QN327L) sorbitol (UNII: 506T60A25R) FD&C red no. 40 (UNII: WZB9127XOA) D&C yellow no. 10 (UNII: 35SW5USQ3G) water (UNII: 059QF0KO0R) Product Characteristics Color orange Score no score Shape OVAL (oval, soft gelatin capsule ) Size 9mm Flavor Imprint Code SPI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35356-500-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021908 06/01/2011 Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) Establishment Name Address ID/FEI Business Operations Lake Erie Medical DBA Quality Care Products LLC 831276758 relabel(35356-500)


