ISOVUE 250- iopamidol injection, solution
ISOVUE 300- iopamidol injection, solution
ISOVUE 370- iopamidol injection, solution
Bracco Diagnostics Inc
----------
ISOVUE®
Pharmacy Bulk Package-
Not for Direct Infusion
ISOVUE®-250
Iopamidol Injection 51%
ISOVUE®-300
Iopamidol Injection 61%
ISOVUE®-370
Iopamidol Injection 76%
ISOVUE
250, 300 and 370 are NOT FOR INTRATHECAL USE.
See Indications, and Dosage and Administration sections for further details on proper use
DIAGNOSTIC NONIONIC RADIOPAQUE CONTRAST MEDIA
For Angiography Throughout the Cardiovascular
System,
Including Cerebral and Peripheral Arteriography,
Coronary
Arteriography and Ventriculography,
Pediatric Angiocardiography,
Selective Visceral
Arteriography and Aortography,
Peripheral Venography (Phlebography), and
Adult and Pediatric
Intravenous Excretory Urography
DESCRIPTION
ISOVUE (Iopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a suitable transfer device.
Each mL of ISOVUE-250 (Iopamidol Injection 51%) provides 510 mg iopamidol with 1 mg tromethamine and 0.33 mg edetate calcium disodium. The solution contains approximately 0.036 mg (0.002 mEq) sodium and 250 mg organically bound iodine per mL.
Each mL of ISOVUE-300 (Iopamidol Injection 61%) provides 612 mg iopamidol with 1 mg tromethamine and 0.39 mg edetate calcium disodium. The solution contains approximately 0.043 mg (0.002 mEq) sodium and 300 mg organically bound iodine per mL.
Each mL of ISOVUE-370 (Iopamidol Injection 76%) provides 755 mg iopamidol with 1 mg tromethamine and 0.48 mg edetate calcium disodium. The solution contains approximately 0.053 mg (0.002 mEq) sodium and 370 mg organically bound iodine per mL.
The pH of ISOVUE contrast media has been adjusted to 6.5-7.5 with hydrochloric acid and/or sodium hydroxide. Pertinent physicochemical data are noted below. ISOVUE (Iopamidol Injection) is hypertonic as compared to plasma and cerebrospinal fluid (approximately 285 and 301 mOsm/kg water, respectively).
| Iopamidol | |||
|---|---|---|---|
| Parameter | 51% | 61% | 76% |
| Concentration (mgI/mL) | 250 | 300 | 370 |
| Osmolality @ 37° C (mOsm/kg water) | 524 | 616 | 796 |
| Viscosity (cP) @ 37° C | 3.0 | 4.7 | 9.4 |
| @ 20° C | 5.1 | 8.8 | 20.9 |
| Specific Gravity @ 37° C | 1.281 | 1.339 | 1.405 |
Iopamidol is designated chemically as (S)-N,N’-bis[2-hydroxy-1-(hydroxymethyl)-ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide. Structural formula:
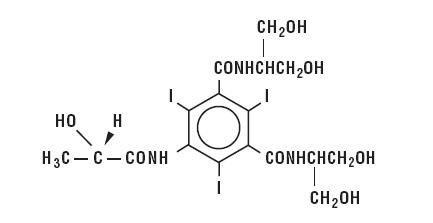 | MW 777.09 C17H22I3N3O8 CAS-60166-93-0 Organically Bound Iodine: 49% |
CLINICAL PHARMACOLOGY
Intravascular injection of a radiopaque diagnostic agent opacifies those vessels in the path of flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs.
Following intravascular injection, radiopaque diagnostic agents are immediately diluted in the circulating plasma. Calculations of apparent volume of distribution at steady-state indicate that iopamidol is distributed between the circulating blood volume and other extracellular fluid; there appears to be no significant deposition of iopamidol in tissues. Uniform distribution of iopamidol in extracellular fluid is reflected by its demonstrated utility in contrast enhancement of computed tomographic imaging of the head and body following intravenous administration.
The pharmacokinetics of intravenously administered iopamidol in normal subjects conform to an open two-compartment model with first order elimination (a rapid alpha phase for drug distribution and a slow beta phase for drug elimination). The elimination serum or plasma half-life is approximately two hours; the half-life is not dose dependent. No significant metabolism, deiodination, or biotransformation occurs.
Iopamidol is excreted mainly through the kidneys following intravascular administration. In patients with impaired renal function, the elimination half-life is prolonged dependent upon the degree of impairment. In the absence of renal dysfunction, the cumulative urinary excretion for Iopamidol, expressed as a percentage of administered intravenous dose is approximately 35 to 40 percent at 60 minutes, 80 to 90 percent at 8 hours, and 90 percent or more in the 72- to 96-hour period after administration. In normal subjects, approximately one percent or less of the administered dose appears in cumulative 72- to 96-hour fecal specimens.
ISOVUE may be visualized in the renal parenchyma within 30-60 seconds following rapid intravenous administration. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1 to 3 minutes, with optimum contrast occurring between 5 and 15 minutes. In patients with renal impairment, contrast visualization may be delayed.
Iopamidol displays little tendency to bind to serum or plasma proteins.
No evidence of in vivo complement activation has been found in normal subjects.
Animal studies indicate that iopamidol does not cross the blood-brain barrier to any significant extent following intravascular administration.
ISOVUE (Iopamidol Injection) enhances computed tomographic brain imaging through augmentation of radiographic efficiency. The degree of enhancement of visualization of tissue density is directly related to the iodine content in an administered dose; peak iodine blood levels occur immediately following rapid injection of the dose. These levels fall rapidly within five to ten minutes. This can be accounted for by the dilution in the vascular and extracellular fluid compartments which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached in about ten minutes; thereafter, the fall becomes exponential. Maximum contrast enhancement frequently occurs after peak blood iodine levels are reached. The delay in maximum contrast enhancement can range from five to forty minutes depending on the peak iodine levels achieved and the cell type of the lesion. This lag suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine within the lesion and outside the blood pool, although the mechanism by which this occurs is not clear. The radiographic enhancement of nontumoral lesions, such as arteriovenous malformations and aneurysms, is probably dependent on the iodine content of the circulating blood pool.
In CECT head imaging, ISOVUE (Iopamidol Injection) does not accumulate in normal brain tissue due to the presence of the “blood-brain” barrier. The increase in x-ray absorption in normal brain is due to the presence of contrast agent within the blood pool. A break in the blood-brain barrier such as occurs in malignant tumors of the brain allows the accumulation of the contrast medium within the interstitial tissue of the tumor. Adjacent normal brain tissue does not contain the contrast medium.
In nonneural tissues (during computed tomography of the body), iopamidol diffuses rapidly from the vascular into the extravascular space. Increase in x-ray absorption is related to blood flow, concentration of the contrast medium, and extraction of the contrast medium by interstitial tissue of tumors since no barrier exists. Contrast enhancement is thus due to the relative differences in extravascular diffusion between normal and abnormal tissue, quite different from that in the brain.
The pharmacokinetics of iopamidol in both normal and abnormal tissue have been shown to be variable. Contrast enhancement appears to be greatest soon after administration of the contrast medium, and following intraarterial rather than intravenous administration. Thus, greatest enhancement can be detected by a series of consecutive two- to three-second scans performed just after injection (within 30 to 90 seconds), i.e., dynamic computed tomographic imaging.
INDICATIONS AND USAGE
ISOVUE (Iopamidol Injection) is indicated for angiography throughout the cardiovascular system, including cerebral and peripheral arteriography, coronary arteriography and ventriculography, pediatric angiocardiography, selective visceral arteriography and aortography, peripheral venography (phlebography), and adult and pediatric intravenous excretory urography.
WARNINGS
Severe Adverse Events-lnadvertent Intrathecal Administration
Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use.
These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that this drug product is not inadvertently administered intrathecally.
General
Nonionic iodinated contrast media inhibit blood coagulation, in vitro, less than ionic contrast media. Clotting has been reported when blood remains in contact with syringes containing nonionic contrast media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state, and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/or three way stopcocks, frequent catheter flushing with heparinized saline solutions, and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
Caution must be exercised in patients with severely impaired renal function, those with combined renal and hepatic disease, or anuria, particularly when larger or repeat doses are administered.
Radiopaque diagnostic contrast agents are potentially hazardous in patients with multiple myeloma or other paraproteinemia, particularly in those with therapeutically resistant anuria. Myeloma occurs most commonly in persons over age 40. Although neither the contrast agent nor dehydration has been proved separately to be the cause of anuria in myelomatous patients, it has been speculated that the combination of both may be causative. The risk in myelomatous patients is not a contraindication; however, special precautions are required.
Contrast media may promote sickling in individuals who are homozygous for sickle cell disease when injected intravenously or intraarterially.
Administration of radiopaque materials to patients known or suspected of having pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure and measures for treatment of a hypertensive crisis should be available. These patients should be monitored very closely during contrast enhanced procedures.
Reports of thyroid storm following the use of iodinated radiopaque diagnostic agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule suggest that this additional risk be evaluated in such patients before use of any contrast medium.
Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age: Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast media in pediatric patients 0 to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
Severe Cutaneous Adverse Reactions: Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering ISOVUE to patients with a history of a severe cutaneous adverse reaction to ISOVUE.
PRECAUTIONS
General
Diagnostic procedures which involve the use of any radiopaque agent should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the particular procedure to be performed. Appropriate facilities should be available for coping with any complication of the procedure, as well as for emergency treatment of severe reaction to the contrast agent itself. After parenteral administration of a radiopaque agent, competent personnel and emergency facilities should be available for at least 30 to 60 minutes since severe delayed reactions may occur. Caution should be exercised in hydrating patients with underlying conditions that may be worsened by fluid overload, such as congestive heart failure.
Preparatory dehydration is dangerous and may contribute to acute renal failure in patients with advanced vascular disease, diabetic patients, and in susceptible nondiabetic patients (often elderly with pre-existing renal disease). Patients should be well hydrated prior to and following iopamidol administration.
The possibility of a reaction, including serious, life-threatening, fatal, anaphylactoid or cardiovascular reactions, should always be considered (see ADVERSE REACTIONS). Patients at increased risk include those with a history of a previous reaction to a contrast medium, patients with a known sensitivity to iodine per se, and patients with a known clinical hypersensitivity (bronchial asthma, hay fever, and food allergies). The occurrence of severe idiosyncratic reactions has prompted the use of several pretesting methods. However, pretesting cannot be relied upon to predict severe reactions and may itself be hazardous for the patient. It is suggested that a thorough medical history with emphasis on allergy and hypersensitivity, prior to the injection of any contrast medium, may be more accurate than pretesting in predicting potential adverse reactions. A positive history of allergies or hypersensitivity does not arbitrarily contraindicate the use of a contrast agent where a diagnostic procedure is thought essential, but caution should be exercised. Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions in such patients should be considered. Recent reports indicate that such pretreatment does not prevent serious life-threatening reactions but may reduce both their incidence and severity.
Pre-existing conditions, such as pacemakers or cardiac medications, specifically beta-blockers, may mask or alter the signs or symptoms of an anaphylactoid reaction, as well as masking or altering the response to particular medications used for treatment. For example, beta-blockers inhibit a tachycardiac response, and can lead to the incorrect diagnosis of a vasovagal rather than an anaphylactoid reaction. Special attention to this possibility is particularly critical in patients suffering from serious, life-threatening reactions.
General anesthesia may be indicated in the performance of some procedures in selected patients; however, a higher incidence of adverse reactions has been reported with radiopaque media in anesthetized patients, which may be attributable to the inability of the patient to identify untoward symptoms, or to the hypotensive effect of anesthesia which can reduce cardiac output and increase the duration of exposure to the contrast agent.
Even though the osmolality of iopamidol is low compared to diatrizoate or iothalamate based ionic agents of comparable iodine concentration, the potential transitory increase in the circulatory osmotic load in patients with congestive heart failure requires caution during injection. These patients should be observed for several hours following the procedure to detect delayed hemodynamic disturbances. Injection site pain and swelling may occur. In the majority of cases it is due to extravasation of contrast medium. Reactions are usually transient and recover without sequelae. However, inflammation and even skin necrosis have been seen on very rare occasions.
In angiographic procedures, the possibility of dislodging plaques or damaging or perforating the vessel wall, or inducing vasospasm, and or subsequent ischemic events, should be borne in mind during catheter manipulations and contrast medium injection. Test injections to ensure proper catheter placement are suggested.
Selective coronary arteriography should be performed only in selected patients and those in whom the expected benefits outweigh the procedural risk. The inherent risks of angiocardiography in patients with chronic pulmonary emphysema must be weighed against the necessity for performing this procedure. Angiography should be avoided whenever possible in patients with homocystinuria, because of the risk of inducing thrombosis and embolism.
See also Pediatric Use.
In addition to the general precautions previously described, special care is required when venography is performed in patients with suspected thrombosis, phlebitis, severe ischemic disease, local infection or a totally obstructed venous system.
Extreme caution during injection of contrast media is necessary to avoid extravasation and fluoroscopy is recommended. This is especially important in patients with severe arterial or venous disease.
Information for Patients
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease, or known thyroid disorder (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food, or if you had any reactions to previous injections of substances used for x-ray procedures (see PRECAUTIONS-General).
- Inform your physician about any other medications you are currently taking, including nonprescription drugs, before you have this procedure.
- Advise patients to inform their physician if they develop a rash after receiving ISOVUE.
- Advise parents/caregivers about the risk of developing thyroid dysfunction after ISOVUE administration. Advise parents/caregivers about when to seek medical care for their child to monitor for thyroid function (see WARNINGS).
Drug Interactions
Renal toxicity has been reported in a few patients with liver dysfunction who were given oral cholecystographic agents followed by intravascular contrast agents. Administration of intravascular agents should therefore be postponed in any patient with a known or suspected hepatic or biliary disorder who has recently received a cholecystographic contrast agent.
Drug/Laboratory Test Interactions
The results of PBI and radioactive iodine uptake studies, which depend on iodine estimations, will not accurately reflect thyroid function for up to 16 days following administration of iodinated contrast media. However, thyroid function tests not depending on iodine estimations, e.g., T3 resin uptake and total or free thyroxine (T4) assays are not affected.
Any test which might be affected by contrast media should be performed prior to administration of the contrast medium.
Laboratory Test Findings
In vitro studies with animal blood showed that many radiopaque contrast agents, including iopamidol, produced a slight depression of plasma coagulation factors including prothrombin time, partial thromboplastin time, and fibrinogen, as well as a slight tendency to cause platelet and/or red blood cell aggregation (see PRECAUTIONS-General).
Transitory changes may occur in red cell and leucocyte counts, serum calcium, serum creatinine, serum glutamic oxaloacetic transaminase (SGOT), and uric acid in urine; transient albuminuria may occur.
These findings have not been associated with clinical manifestations.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. No evidence of genetic toxicity was obtained in in vitro tests.
Pregnancy: Teratogenic
Effects
Reproduction
studies have been performed in rats and rabbits at doses up to 2.7
and 1.4 times the maximum recommended human dose (1.48 gI/kg in a
50 kg individual), respectively, and have revealed no evidence of
impaired fertility or harm to the fetus due to iopamidol. There are,
however, no adequate and well-controlled studies in pregnant women.
Because animal reproduction studies are not always predictive of human
response, this drug should be used during pregnancy only if clearly
needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when iopamidol is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children has been established in pediatric angiocardiography and excretory urography. Pediatric patients at higher risk of experiencing adverse events during contrast medium administration may include those having asthma, a sensitivity to medication and/or allergens, cyanotic heart disease, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age.
Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates; some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 to 3 years of age based on underlying risk factors, especially in term and preterm neonates (see WARNINGS and ADVERSE REACTIONS).
ADVERSE REACTIONS
Adverse reactions following the use of iopamidol are usually mild to moderate, self-limited, and transient.
In angiocardiography (597 patients), the adverse reactions with an estimated incidence of one percent or higher are: hot flashes 3.4%; angina pectoris 3.0%; flushing 1.8%; bradycardia 1.3%; hypotension 1.0%; hives 1.0%.
In a clinical trial with 76 pediatric patients undergoing angiocardiography, 2 adverse reactions (2.6%) both remotely attributed to the contrast media were reported. Both patients were less than 2 years of age, both had cyanotic heart disease with underlying right ventricular abnormalities and abnormal pulmonary circulation. In one patient pre-existing cyanosis was transiently intensified following contrast media administration. In the second patient pre-existing decreased peripheral perfusion was intensified for 24 hours following the examination. (See “PRECAUTIONS” Section for information on high risk nature of these patients.)
Intravascular injection of contrast media is frequently associated with the sensation of warmth and pain especially in peripheral arteriography and venography; pain and warmth are less frequent and less severe with ISOVUE (Iopamidol Injection) than with diatrizoate meglumine and diatrizoate sodium injection.
The following table of incidence of reactions is based on clinical studies with ISOVUE in about 2246 patients.
| Adverse Reactions | ||
| Estimated Overall Incidence | ||
| System | > 1% | ≤ 1% |
| Cardiovascular | none | tachycardia hypotension hypertension myocardial ischemia circulatory collapse S-T segment depression bigeminy extrasystoles ventricular fibrillation angina pectoris bradycardia transient ischemic attack thrombophlebitis |
| Nervous | pain (2.8%) burning sensation (1.4%) | vasovagal reaction tingling in arms grimace faintness |
| Digestive | nausea (1.2%) | vomiting anorexia |
| Respiratory | none | throat constriction dyspnea pulmonary edema |
| Skin and Appendages | none | rash urticaria pruritus flushing |
| Body as a Whole | hot flashes (1.5%) | headache fever chills excessive sweating back spasm |
| Special Senses | warmth (1.1%) | taste alterations nasal congestion visual disturbances |
| Urogenital | none | urinary retention |
Regardless of the contrast agent employed, the overall estimated incidence of serious adverse reactions is higher with coronary arteriography than with other procedures. Cardiac decompensation, serious arrhythmias, or myocardial ischemia or infarction have been reported with ISOVUE and may occur during coronary arteriography and left ventriculography.
Following coronary and ventricular injections, certain electrocardiographic changes (increased QTc, increased R-R, T-wave amplitude) and certain hemodynamic changes (decreased systolic pressure) occurred less frequently with ISOVUE (Iopamidol Injection) than with diatrizoate meglumine and diatrizoate sodium injection; increased LVEDP occurred less frequently after ventricular iopamidol injections.
In aortography, the risks of procedures also include injury to the aorta and neighboring organs, pleural puncture, renal damage including infarction and acute tabular necrosis with oliguria and anuria, accidental selective filling of the right renal artery during the translumbar procedure in the presence of preexisting renal disease, retroperitoneal hemorrhage from the translumbar approach, and spinal cord injury and pathology associated with the syndrome of transverse myelitis.
The following adverse reactions have been reported for Iopamidol: Cardiovascular: arrhythmia, arterial spasms, flushing, vasodilation, chest pain, cardiopulmonary arrest; Nervous System: confusion, paresthesia, dizziness, temporary cortical blindness, temporary amnesia, convulsions, paralysis, coma; Respiratory: increased cough, sneezing, asthma, apnea, laryngeal edema, chest tightness, rhinitis; Skin and Appendages: injection site pain usually due to extravasation and/or erythematous swelling, pallor, periorbital edema, facial edema; Urogenital: pain, hematuria; Special Senses: watery itchy eyes, lacrimation, conjunctivitis; Musculoskeletal: muscle spasm, involuntary leg movement; Body as a whole: tremors, malaise, anaphylactoid reaction (characterized by cardiovascular, respiratory and cutaneous symptoms), pain; Digestive: severe retching and choking, abdominal cramps. Some of these may occur as a consequence of the procedure. Other reactions may also occur with the use of any contrast agent as a consequence of the procedural hazard; these include hemorrhage or pseudoaneurysms at the puncture site, brachial plexus palsy following axillary artery injections, chest pain, myocardial infarction, and transient changes in hepatorenal chemistry tests. Arterial thrombosis, displacement of arterial plaques, venous thrombosis, dissection of the coronary vessels and transient sinus arrest are rare complications.
General Adverse Reactions To Contrast Media
Reactions known to occur with parenteral administration of iodinated ionic contrast agents (see the listing below) are possible with any nonionic agent. Approximately 95 percent of adverse reactions accompanying the use of other water-soluble intravascularly administered contrast agents are mild to moderate in degree. However, life-threatening reactions and fatalities, mostly of cardiovascular origin, have occurred. Reported incidences of death from the administration of other iodinated contrast media range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Most deaths occur during injection or 5 to 10 minutes later, the main feature being cardiac arrest with cardiovascular disease as the main aggravating factor. Isolated reports of hypotensive collapse and shock are found in the literature. The incidence of shock is estimated to be 1 out of 20,000 (0.005 percent) patients.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions. Chemotoxic reactions result from the physicochemical properties of the contrast medium, the dose, and the speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category. Experience with iopamidol suggests there is much less discomfort (e.g. pain and/or warmth) with peripheral arteriography. Fewer changes are noted in ventricular function after ventriculography and coronary arteriography.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of drug injected, the speed of injection, the mode of injection, and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate, and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
The reported incidence of adverse reactions to contrast media in patients with a history of allergy is twice that for the general population. Patients with a history of previous reactions to a contrast medium are three times more susceptible than other patients. However, sensitivity to contrast media does not appear to increase with repeated examinations. Most adverse reactions to intravascular contrast agents appear within one to three minutes after the start of injection, but delayed reactions may occur. Delayed reactions, usually involving the skin, may uncommonly occur within 2-3 days (range 1-7 days) after the administration of contrast (see PRECAUTIONS-General). Delayed allergic reactions are more frequent in patients treated with immunostimulants, such as interleukin-2.
In addition to the adverse drug reactions reported for iopamidol, the following additional adverse reactions have been reported with the use of other intravascular contrast agents and are possible with the use of any water-soluble iodinated contrast agent: Cardiovascular: cerebral hematomas, petechiae; Hematologic: neutropenia; Urogenital: osmotic nephrosis of proximal tubular cells, renal failure; Special Senses: conjunctival chemosis with infection; Endocrine: hyperthyroidism, hypothyroidism; Skin and Subcutaneous Tissue Disorders: Skin necrosis; Reactions range from mild (e.g. rash, erythema, pruritus, urticaria and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)].
OVERDOSAGE
Treatment of an overdose of an injectable radiopaque contrast medium is directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
DOSAGE AND ADMINISTRATION
General
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
It is desirable that solutions of radiopaque diagnostic agents for intravascular use be at body temperature when injected. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Sterile techniques must be used with any intravascular injection, and with catheters and guidewires.
The transferring of ISOVUE from ISOVUE Pharmacy Bulk Package should be performed in a suitable work area, such as a laminar flow hood, utilizing aseptic technique. The container closure may be penetrated only one time, utilizing a suitable transfer device.
Patients should be well hydrated prior to and following ISOVUE (Iopamidol Injection) administration.
As with all radiopaque contrast agents, only the lowest dose of ISOVUE necessary to obtain adequate visualization should be used. A lower dose reduces the possibility of an adverse reaction. Most procedures do not require use of either a maximum dose or the highest available concentration of ISOVUE; the combination of dose and ISOVUE concentration to be used should be carefully individualized, and factors such as age, body size, size of the vessel and its blood flow rate, anticipated pathology and degree and extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed should be considered.
Cerebral Arteriography
ISOVUE-300 (Iopamidol Injection, 300 mgI/mL) should be used. The usual individual injection by carotid puncture or transfemoral catheterization is 8 to 12 mL, with total multiple doses ranging to 90 mL.
Peripheral Arteriography
ISOVUE-300 usually provides adequate visualization. For injection into the femoral artery or subclavian artery, 5 to 40 mL may be used; for injection into the aorta for a distal runoff, 25 to 50 mL may be used. Doses up to a total of 250 mL of ISOVUE-300 have been administered during peripheral arteriography.
Selective Visceral Arteriography and Aortography
ISOVUE-370 (Iopamidol Injection, 370 mgI/mL) should be used. Doses up to 50 mL may be required for injection into the larger vessels such as the aorta or celiac artery; doses up to 10 mL may be required for injection into the renal arteries. Often, lower doses will be sufficient. The combined total dose for multiple injections has not exceeded 225 mL.
Pediatric Angiocardiography
ISOVUE-370 should be used. Pediatric angiocardiography may be performed by injection into a large peripheral vein or by direct catheterization of the heart.
The usual dose range for single injections is provided in the following table:
| Single Injection | |
| Usual Dose Range | |
| Age | mL |
| < 2 years | 10-15 |
| 2-9 years | 15-30 |
| 10-18 years | 20-50 |
The usual recommended dose for cumulative injections is provided in the following table:
| Cumulative Injection | |
| Usual Recommended Dose | |
| Age | mL |
| < 2 years | 40 |
| 2-4 years | 50 |
| 5-9 years | 100 |
| 10-18 years | 125 |
Coronary Arteriography and Ventriculography
ISOVUE-370 should be used. The usual dose for selective coronary artery injections is 2 to 10 mL. The usual dose for ventriculography, or for nonselective opacification of multiple coronary arteries following injection at the aortic root is 25 to 50 mL. The total dose for combined procedures has not exceeded 200 mL. EKG monitoring is essential.
Excretory Urography
ISOVUE-250 ISOVUE-300 or ISOVUE-370 may be used. The usual adult dose for ISOVUE-250 is 50 to 100 mL, for ISOVUE-300 is 50 mL and for ISOVUE-370 is 40 mL administered by rapid intravenous injection.
DRUG HANDLING
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
Directions for Proper Use of ISOVUE Pharmacy Bulk Package
The Pharmacy Bulk Package is used as a multiple dose container with an appropriate transfer device to fill empty sterile syringes.
ISOVUE injection should be drawn into the syringe and administered using sterile technique. Unused portions of the drug must be discarded.
- The transferring ISOVUE (Iopamidol Injection) from the Pharmacy Bulk Package should be performed in a suitable work area, such as a laminar flow hood, utilizing aseptic technique.
- The container closure may be penetrated only one time, utilizing a suitable transfer device. Once the Pharmacy Bulk Package is punctured, it should not be removed from the aseptic work area during the entire period of use.
- The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of 10 hours from initial closure entry is permitted to complete fluid transfer operation. Any unused ISOVUE Pharmacy Bulk Package injection must be discarded 10 hours after initial puncture of the bulk package.
- Storage temperature of container after the closure has been entered should not exceed 25° C (77° F).
HOW SUPPLIED
ISOVUE-250 (Iopamidol Injection 51%)
Ten 200 mL Pharmacy Bulk Packages (NDC 0270-1317-41)
| ISOVUE
250
iopamidol injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ISOVUE
300
iopamidol injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ISOVUE
370
iopamidol injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bracco Diagnostics Inc (849234661) |
| Registrant - Bracco Diagnostics Inc (849234661) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BRACCO IMAGING SPA | 434384007 | API MANUFACTURE(0270-1316, 0270-1317, 0270-1315) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BIPSO GmbH | 342104149 | MANUFACTURE(0270-1316, 0270-1315, 0270-1317) , ANALYSIS(0270-1316, 0270-1317, 0270-1315) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Labor LS SE & Co. KG | 314929072 | ANALYSIS(0270-1316, 0270-1317, 0270-1315) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Italia S.p.A | 434078638 | MANUFACTURE(0270-1315, 0270-1316, 0270-1317) , ANALYSIS(0270-1316, 0270-1317, 0270-1315) | |


