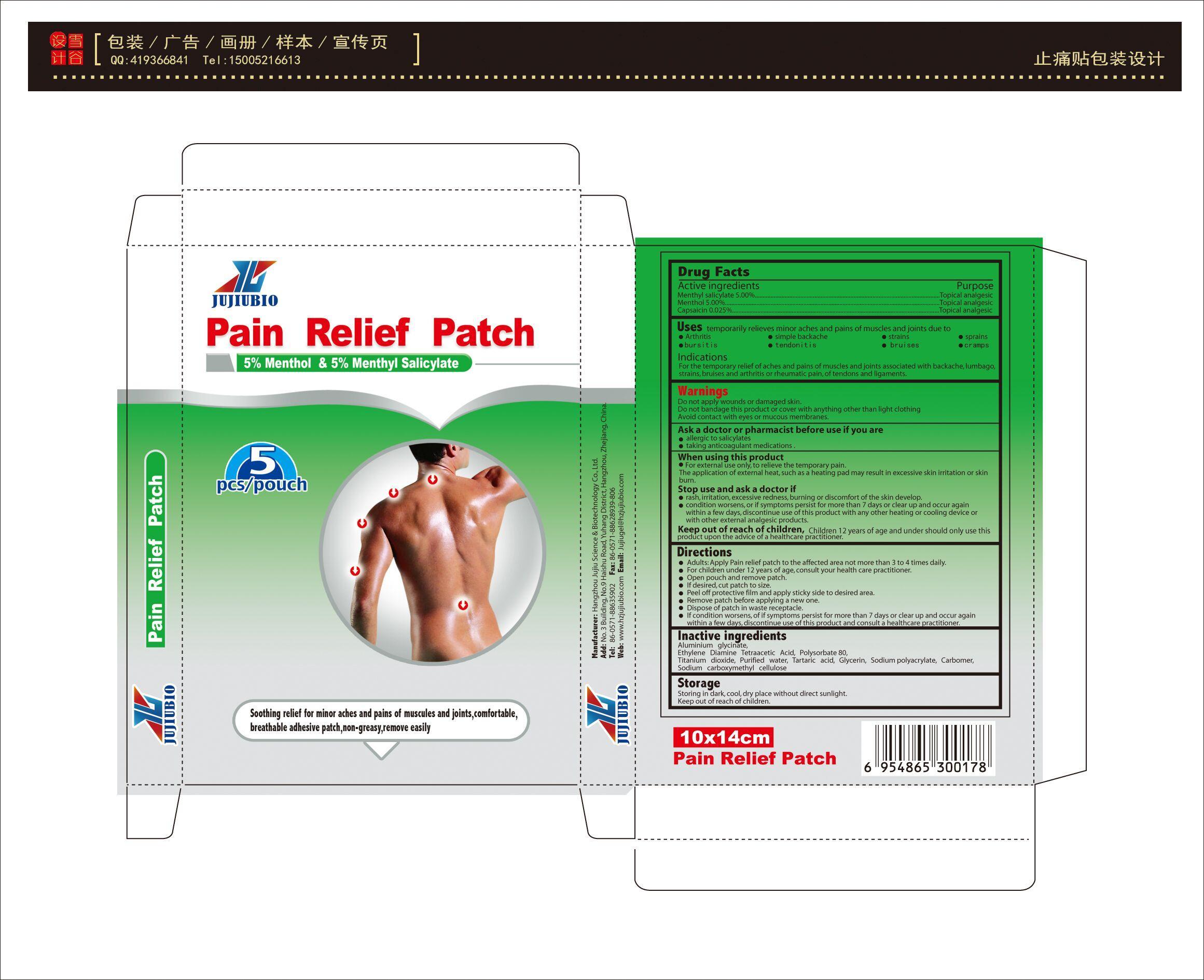
Label: PAIN RELIEF PATCH- menthol,capsaicin,menthyl salicylate patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 69963-001-01 - Packager: Hangzhou Jujiu Science & Biotechnology Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnning
Do not apply wounds or damaged skin.
Do not bandage this product or cover with anything other than light clothing
Avoid contact with eyes or mucous membranes.
Ask a doctor or pharmacist before use if you are
allergic to salicylates
taking anticoagulant medications .
When using this product
For external use only, to relieve the temporary pain.
The application of external heat, such as a heating pad may result in excessive skin irritation or skin burn.
Stop use and ask a doctor if
rash, irritation, excessive redness, burning or discomfort of the skin develop.
condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product with any other heating or cooling device or with other external analgesic products. -
Directions
Adults: Apply Pain relief patch to the affected area not more than 3 to 4 times daily.
For children under 12 years of age, consult your health care practitioner.
Open pouch and remove patch.
If desired, cut patch to size.
Peel off protective film and apply sticky side to desired area.
Remove patch before applying a new one.
Dispose of patch in waste receptacle.
If condition worsens, of if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a healthcare practitioner. - Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PATCH
menthol,capsaicin,menthyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69963-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g MENTHYL SALICYLATE, (+/-)- (UNII: 43XOA705ZD) (SALICYLIC ACID - UNII:O414PZ4LPZ) MENTHYL SALICYLATE, (+/-)- 5 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength ALUMINIUM TRIGLYCINATE (UNII: 5TLG1CL557) 0.025 g in 100 g DIAMINOHYDROXYPROPANETETRAACETIC ACID (UNII: 949B9ZMO7M) 0.025 g in 100 g POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.025 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.025 g in 100 g WATER (UNII: 059QF0KO0R) 0.025 g in 100 g TARTARIC ACID (UNII: W4888I119H) 0.025 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) 0.025 g in 100 g SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) 0.025 g in 100 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.025 g in 100 g CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 0.025 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69963-001-01 5 in 1 POUCH 01/08/2018 1 0.5 g in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/10/2015 Labeler - Hangzhou Jujiu Science & Biotechnology Co.,Ltd. (543207110) Registrant - Hangzhou Jujiu Science & Biotechnology Co.,Ltd. (543207110) Establishment Name Address ID/FEI Business Operations Hangzhou Jujiu Science & Biotechnology Co.,Ltd. 543207110 manufacture(69963-001)