Label: KETOFEN- ketoprofen injection, solution
- NDC Code(s): 54771-4396-1, 54771-4396-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
Drug Label Information
Updated March 31, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Ketoprofen is a non-steroidal anti-inflammatory agent of the propionic acid class that includes ibuprofen, naproxen, and fenoprofen. Active Ingredient: Each mL contains 100 mg ketoprofen/mL of aqueous solution. Inactive Ingredients: 70 mg L-Arginine/mL; citric acid (to adjust pH); benzyl alcohol, 0.025 g (as preservative).
It is packaged in a multiple dose bottle. - INDICATION
- DOSAGE AND ADMINISTRATION
- CONTRAINDICATIONS
- USER SAFETY WARNINGS
- OTHER WARNINGS
-
PRECAUTIONS
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Consider stopping therapy if adverse reactions, such as prolonged inappetence or abnormal feces, could be attributed to gastrointestinal toxicity. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached. Since many NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforation, concomitant use of ketoprofen with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided or closely monitored.
Studies to determine activity of KETOFEN when administered concomitantly with other drugs have not been conducted. Drug compatibility should be monitored closely in patients requiring
adjunctive therapy.
This product should not be used in breeding animals since the effects of KETOFEN on fertility, pregnancy or fetal health in horses have not been determined. - SIDE EFFECTS
- CONTACT INFORMATION
-
PHARMACOLOGY
KETOFEN is a non-narcotic, non-steroidal anti-inflammatory agent with analgesic and antipyretic properties.
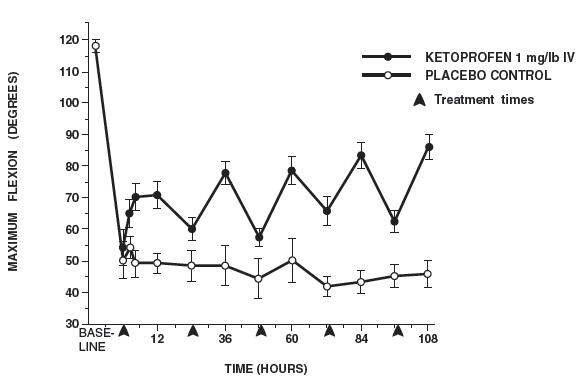
In horses, intravenous dosages of ketoprofen ranging from 0.5 to 1.5 mg/lb resulted in dosage dependent anti-inflammatory effects in the chronic adjuvant carpitis model as depicted in the following graph.n = number of animals - *
- sem = standard error of the mean
MAXIMUM FLEXION (intravenous ketoprofen, mean ± sem, n = 4)* 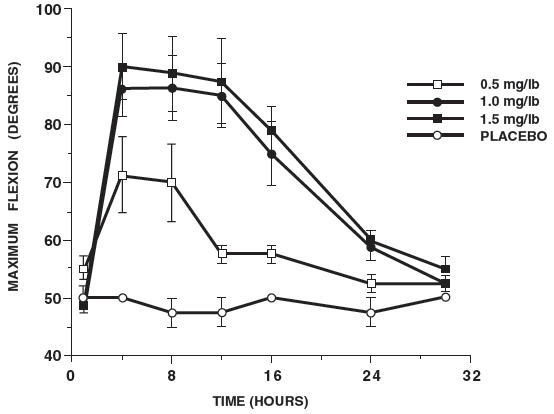
Additional studies using the same model in horses have shown that the effects of ketoprofen are maximal by 12 hours and still measurable at 24 hours after each dosage as depicted in the following graph.
-
TOXICITY
Horses were found to tolerate ketoprofen given intravenously at dosages of 0, 1, 3 and 5 mg/lb once daily for 15 consecutive days (up to five times the recommended dosage for three times the usual duration) with no evidence of toxic effects. In clinical studies, intravenous injection of 1 mg/lb/day for five days resulted in no injection site irritation or other side effects.
At 15-fold overdose (15 mg/lb/day) for five days one of two horses developed severe laminitis, but no gross lesions or histologic changes were observed. The toxic effects observed in the horses given a 25-fold overdose (25 mg/lb/day) for five days included inappetence, depression, icterus, abdominal swelling and postmortem findings of gastritis, nephritis and hepatitis. - HOW SUPPLIED
- STORAGE CONDITIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Ketoprofen is a non-steroidal anti-inflammatory agent of the propionic acid class that includes ibuprofen, naproxen, and fenoprofen. Active Ingredient: Each mL contains 100 mg ketoprofen/mL of aqueous solution. Inactive Ingredients: 70 mg L-Arginine/mL; citric acid (to adjust pH); benzyl alcohol, 0.025 g (as preservative).
It is packaged in a multiple dose bottle.
-
INDICATIONS
KETOFEN® (ketoprofen) is indicated for the control of pyrexia associated with Bovine Respiratory Disease (BRD) in beef heifers, beef steers, beef calves 2 months of age and older, beef bulls, replacement dairy heifers, and dairy bulls. Not for use in reproducing animals over one year of age, dairy calves, or veal calves. Not for use in lactating dairy cattle or calves <2 months old.
-
DOSAGE AND ADMINISTRATION
Cattle: The recommended dosage is 3 mg/kg (1 mL/33.3 kg) or 1.36 mg/lb (1 mL/74 lb) of body weight. Treatment is administered by subcutaneous injection once daily and may be repeated for up to three days if pyrexia persists.
Use contents within 4 months of first vial puncture.
Cattle Dosing Guide:Animal Weight (lb) Dose Volume (mL) Animal Weight (lb) Dose Volume (mL) 150 2.1 600 8.4 200 2.8 650 9.1 250 3.5 700 9.8 300 4.2 750 10.5 350 4.9 800 11.2 400 5.6 850 11.9 450 6.3 900 12.6 500 7.0 950 13.3 550 7.7 1000 14.0
- CONTRAINDICATIONS
-
WITHDRAWAL PERIODS AND RESIDUE WARNINGS
Cattle must not be slaughtered for human consumption within 48-hours following last treatment with this drug product. Not for use in female dairy cattle 1 year of age or older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows or heifers. Not for use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
- USER SAFETY WARNINGS
- ANIMAL SAFETY WARNINGS
-
PRECAUTIONS
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, hepatic and renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual patient. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction.
Since many NSAIDs possess the potential to induce gastrointestinal ulceration, concomitant use of KETOFEN® with other antiinflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided or closely monitored. Discontinue use if fecal blood is observed.
The effects of KETOFEN® on bovine reproductive performance, pregnancy, lactation, or on animals of reproductive age intended for breeding has not been investigated.
KETOFEN® may cause injection site swelling that appears 1 to 3 days post-treatment and typically resolves by 28 days post-injection. These reactions may result in trim loss of edible tissue at slaughter.
-
ADVERSE REACTIONS
Repeated administration of NSAIDs can result in gastric or renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual patient. Patients at greatest risk for toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with pre-existing gastric ulcers, renal, cardiovascular, and/or hepatic dysfunction.
- CONTACT INFORMATION
-
CLINICAL PHARMACOLOGY
KETOFEN is a non-narcotic, non-steroidal anti-inflammatory agent. The primary mechanism of action for ketoprofen is inhibition of the cyclooxygenase (COX) pathway leading to decreased production of prostaglandins[1]. Ketoprofen is a relatively nonselective inhibitor of the COX isozymes. After subcutaneous administration of a single dose of 3 mg/kg ketoprofen in fourteen calves, the mean and range (minimum and maximum) for the maximum plasma concentration (Cmax) was 9.40 μg/mL (7.06 – 15.8), the area under the curve to the last quantifiable point AUC(last) was 67.4 μg•h/mL (48.4 – 96.9), the time to maximum plasma concentration (Tmax) was 1.54 hours (0.67-2.0), and the half-life (t1/2) was 3.03 hours (2.48-3.88). Ketoprofen has shown linear pharmacokinetics across a 1 to 15 mg/kg dose range and has shown little to no accumulation on multiple dosing with a 24-hour dose interval.
-
EFFECTIVENESS
The control of pyrexia associated with bovine respiratory disease (BRD) was demonstrated in a single multisite study. Cattle exhibiting clinical signs of BRD and having a rectal temperature of at least 104.5°F were enrolled. A total of 202 cattle were administered either a single subcutaneous injection of KETOFEN® (3.0 mg/kg BW) or a single subcutaneous injection of saline (0.028 mL/kg BW) on day 0. Six hours after treatment, rectal temperatures were measured. The treatment success rate of the KETOFEN®-treated group was compared to the treatment success rate in the saline-treated group. A treatment success was defined as a decrease in rectal temperature of ≥ 2°F in an individual animal. The percent of animals with a ≥ 2 degree decrease in rectal temperature at 6 hours post-treatment was significantly different and higher (P=0.0215) in the KETOFEN® group (74.3%) vs. the saline treated group (5.9%).
-
TARGET ANIMAL SAFETY
Margin of Safety: KETOFEN® was injected with a 1X (3 mg/kg BW), 3X (9 mg/kg BW), and 5X (15 mg/kg BW) dose daily for 9 consecutive days (3 times the maximum recommended duration). The only treatment-associated finding present at a 1X (3 mg/kg BW) dose for 9 days was minimal to mild gross and microscopic renal tubular lesions. No ketoprofen-related changes were noted in clinical or general health observations, hematology, urinalysis, fecal occult blood, or serum chemistries in cattle administered a 1X (3 mg/kg BW) dose for 9 days. At a 3X dose, subclinical focal ulcers in the abomasum and minimal to mild renal lesions were present. Cattle injected with a 5X dose of ketoprofen for 9 days had similar treatment related findings, except for one of eight calves which had clinical signs of toxicity, due to peritonitis subsequent to abomasal ulceration on the 9th treatment day. Discontinue treatment if fecal blood is observed. Do not use in cattle that are dehydrated or with known renal disease.
Injection site safety: Eight calves were injected with a 1X dose of ketoprofen daily for 3 consecutive days with clinical observations of injection sites with necropsy on days 7, 14, 28, and 42. Injection of 3 consecutive doses of KETOFEN® resulted in transient injection site reactions palpable through 14 days, with resolution by 28 days post-administration. Injection site lesions included discoloration involving the skin and subcutaneous tissue on Day 7. By Day 42 discoloration was limited to subcutaneous tissue. Subcutaneous injection of ketoprofen can cause a transient local tissue reaction. These reactions may result in trim loss of edible tissue at slaughter.
Field safety: There were no adverse events reported in investigational field studies with the injection of KETOFEN® under the intended conditions of use.
- REFERENCES
- HOW SUPPLIED
- STORAGE CONDITIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 100 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
KETOFEN
ketoprofen injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-4396 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOPROFEN (UNII: 90Y4QC304K) (KETOPROFEN - UNII:90Y4QC304K) KETOPROFEN 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength ARGININE (UNII: 94ZLA3W45F) 70 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-4396-1 1 in 1 CARTON 1 50 mL in 1 BOTTLE 2 NDC:54771-4396-2 1 in 1 CARTON 2 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140269 09/26/1990 Labeler - Zoetis Inc. (828851555)