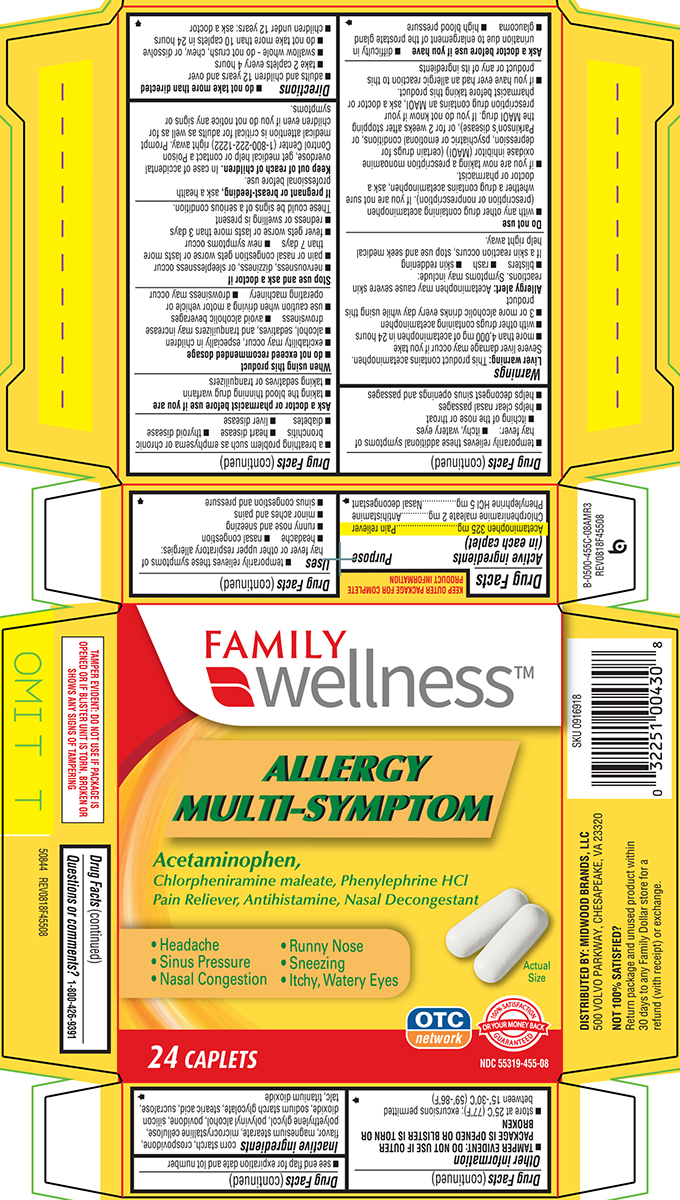
ALLERGY MULTI-SYMPTOM- acetaminophen, chlorpheniramine maleate and phenylephrine hcl tablet, film coated
Family Dollar Services Inc
----------
Family Wellness 44-455C-AMS-Delisted
Active ingredients (in each caplet)
Acetaminophen 325 mg
Chlorpheniramine maleate 2 mg
Phenylephrine HCl 5 mg
Uses
- temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
- headache
- nasal congestion
- runny nose and sneezing
- minor aches and pains
- sinus congestion and pressure
- temporarily relieves these additional symptoms of hay fever:
- itchy, watery eyes
- itching of the nose or throat
- helps clear nasal passages
- helps decongest sinus openings and passages
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
- high blood pressure
- a breathing problem such as emphysema or chronic bronchitis
- heart disease
- thyroid disease
- diabetes
- liver disease
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
- drowsiness may occur
Directions
- do not take more than directed
- adults and children 12 years and over
- take 2 caplets every 4 hours
- swallow whole - do not crush, chew, or dissolve
- do not take more than 10 caplets in 24 hours
- children under 12 years: ask a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, crospovidone, flavor, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, sucralose, talc, titanium dioxide
Principal Display Panel
FAMILY
wellness™
Allergy
Multi-Symptom
Acetaminophen, Chlorpheniramine Maleate, Phenylephrine HCl
Pain Reliever, Antihistamine, Nasal Decongestant
• Headache • Runny Nose
• Sinus Pressure • Sneezing
• Nasal Congestion • Itchy, Watery Eyes
Actual Size
24 CAPLETS
OTC
network
100% SATISFACTION GUARANTEED
OR YOUR MONEY BACK
NDC 55319-455-08
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR
SHOWS ANY SIGNS OF TAMPERING
50844 REV0818F45508
DISTRIBUTED BY: MIDWOOD BRANDS, LLC
500 VOLVO PARKWAY, CHESAPEAKE, VA 23320
NOT 100% SATISFIED?
Return package and unused product within
30 days to any Family Dollar store for a
refund (with receipt) or exchange.
Family Wellness 44-455C
| ALLERGY MULTI-SYMPTOM
acetaminophen, chlorpheniramine maleate and phenylephrine hcl tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Family Dollar Services Inc (024472631) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(55319-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(55319-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(55319-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(55319-455) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(55319-455) | |