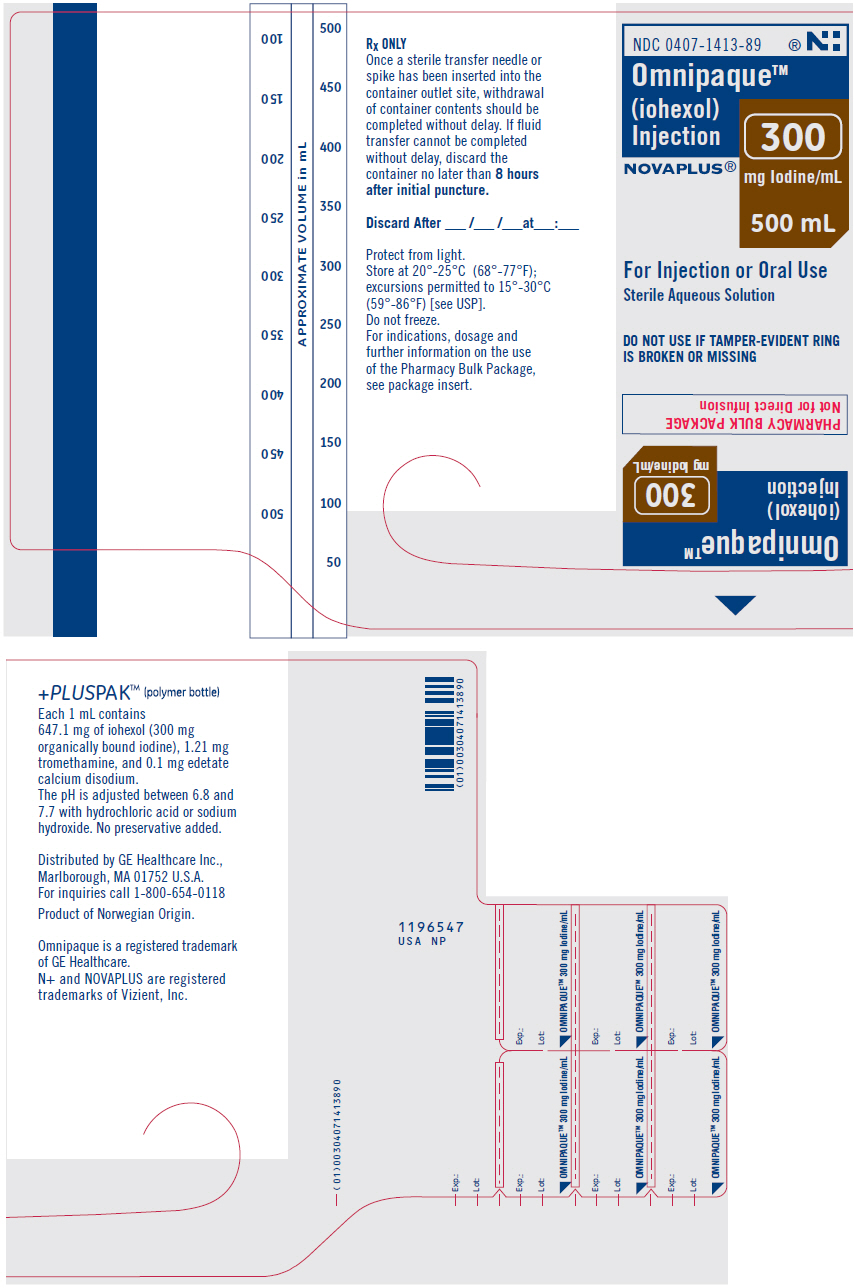
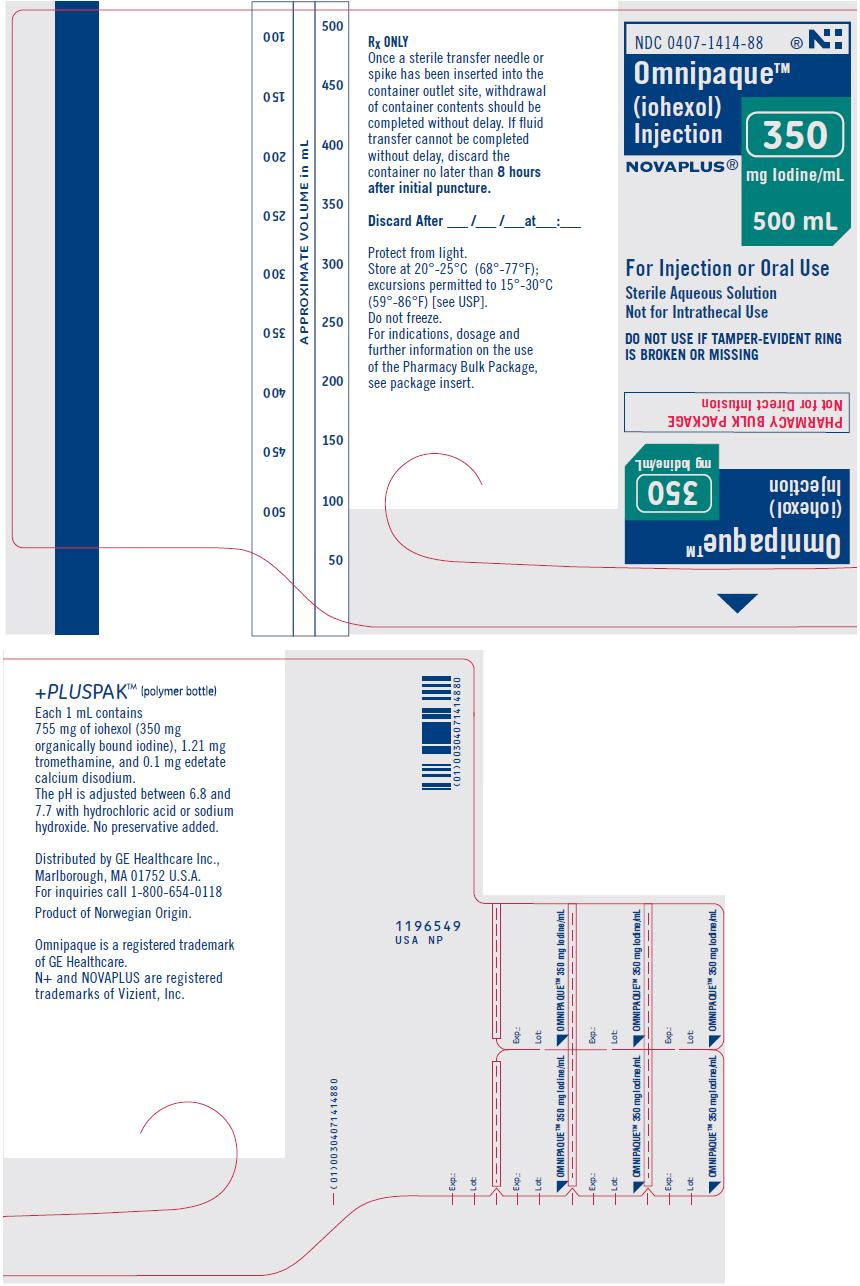
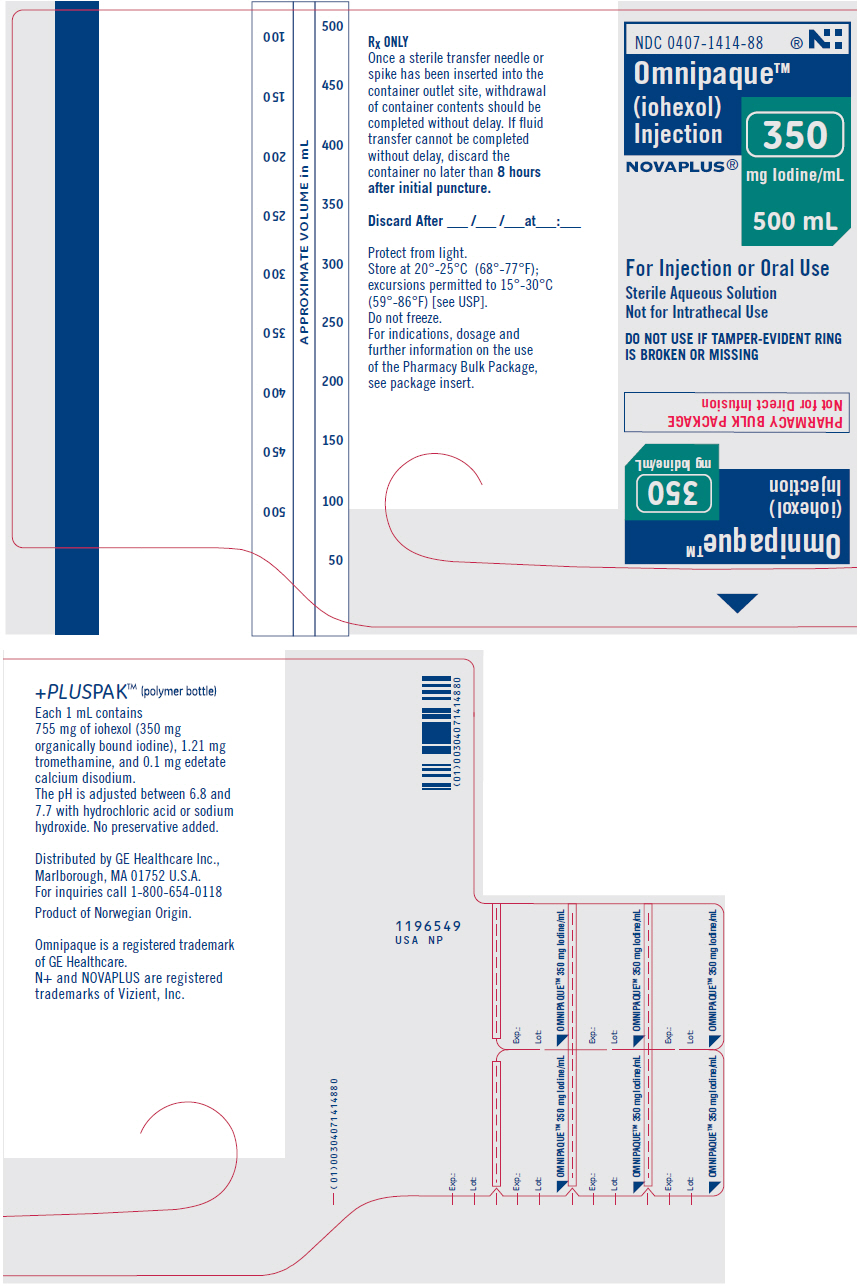
Label: OMNIPAQUE- iohexol injection, solution
- NDC Code(s): 0407-1413-89, 0407-1414-88
- Packager: GE Healthcare Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
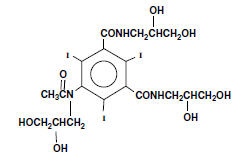
Iohexol, N, N´ - Bis(2,3-dihydroxypropyl)-5-[ N-(2,3-dihydroxypropyl)-acetamido]-2,4,6-triiodoisophthalamide, is a nonionic, water-soluble radiographic contrast medium with a molecular weight of 821.14 (iodine content 46.36%). In aqueous solution each triiodinated molecule remains undissociated. The chemical structure is:
OMNIPAQUE is provided as a sterile, pyrogen-free, colorless to pale-yellow solution, in Pharmacy Bulk Package, in the following iodine concentrations: 300 and 350 mg Iodine/mL. A Pharmacy Bulk Package is used to dispense multiple single doses, utilizing a suitable transfer device. OMNIPAQUE 300 contains 647 mg of iohexol equivalent to 300 mg of organic iodine per mL; and OMNIPAQUE 350 contains 755 mg of iohexol equivalent to 350 mg of organic iodine per mL. Each milliliter of iohexol solution contains 1.21 mg tromethamine and 0.1 mg edetate calcium disodium with the pH adjusted between 6.8 and 7.7 with hydrochloric acid or sodium hydroxide. All solutions are sterilized by autoclaving and contain no preservatives. Iohexol solution is sensitive to light and therefore should be protected from exposure.
The available concentrations have the following physical properties:
Concentration
(mg Iodine/mL)Osmolality *
(mOsm/kg water)Osmolarity
(mOsm/L)Absolute Viscosity
(cp)Specific Gravity 20°C 37°C 37°C - *
- By vapor-pressure osmometry.
300 672 465 11.8 6.3 1.349 350 844 541 20.4 10.4 1.406 OMNIPAQUE 300 and OMNIPAQUE 350 have osmolalities from approximately 2.2 to 3 times that of plasma (285 mOsm/kg water) or cerebrospinal fluid (301 mOsm/kg water) as shown in the above table and are hypertonic under conditions of use.
- SECTION I
-
CLINICAL PHARMACOLOGY—Intrathecal
Iohexol is absorbed from cerebrospinal fluid (CSF) into the bloodstream and is eliminated by renal excretion. No significant metabolism, deiodination, or biotransformation occurs.
The initial concentration and volume of the medium, in conjunction with appropriate patient manipulation and the volume of CSF into which the medium is placed, will determine the extent of the diagnostic contrast that can be achieved.
Following intrathecal injection in conventional radiography, OMNIPAQUE 300 will continue to provide good diagnostic contrast for at least 30 minutes. Slow diffusion of iohexol takes place throughout the CSF with subsequent absorption into the bloodstream. Once in the systemic circulation, iohexol displays little tendency to bind to serum or plasma proteins. At approximately 1 hour following injection, contrast of diagnostic quality will no longer be available for conventional myelography. If computerized tomographic (CT) myelography is to follow, consideration should be given to a delay of several hours to allow the degree of contrast to decrease.
After administration into the lumbar subarachnoid space, computerized tomography shows the presence of contrast medium in the thoracic region in about 1 hour, in the cervical region in about 2 hours, and in the basal cisterns in 3 to 4 hours.
In patients with renal impairment, depending on the degree of impairment, prolonged plasma iohexol levels may be anticipated due to decreased renal elimination.
- INDICATIONS AND USAGE—Intrathecal
-
CONTRAINDICATIONS—Intrathecal
OMNIPAQUE should not be administered to patients with a known hypersensitivity to iohexol. Myelography should not be performed in the presence of significant local or systemic infection where bacteremia is likely. Intrathecal administration of corticosteroids with OMNIPAQUE is contraindicated. Because of the possibility of overdosage, immediate repeat myelography in the event of technical failure is contraindicated (see DOSAGE AND ADMINISTRATION).
-
WARNINGS—General
SEVERE ADVERSE EVENTS - INADVERTENT INTRATHECAL ADMINISTRATION
Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to ensure that OMNIPAQUE 350 is not administered intrathecally. (OMNIPAQUE 300 is approved for intrathecal administration).
If grossly bloody CSF is encountered, the possible benefits of a myelographic procedure should be considered in terms of the risk to the patient.
Caution is advised in patients with a history of epilepsy, severe cardiovascular disease, chronic alcoholism, or multiple sclerosis.
Elderly patients may present a greater risk following myelography. The need for the procedure in these patients should be evaluated carefully. Special attention must be paid to dose and concentration of the medium, hydration, and technique used.
Patients who are receiving anticonvulsants should be maintained on this therapy. Should a seizure occur, intravenous diazepam or phenobarbital sodium is recommended. In patients with a history of seizure activity who are not on anticonvulsant therapy, premedication with barbiturates should be considered.
Prophylactic anticonvulsant treatment with barbiturates should be considered in patients with evidence of inadvertent intracranial entry of a large or concentrated bolus of the contrast medium since there may be an increased risk of seizure in such cases.
Drugs which lower the seizure threshold, especially phenothiazine derivatives, including those used for their antihistamine properties, are not recommended for use with OMNIPAQUE. Drugs which lower the seizure thresh old, especially phenothiazine derivatives, including those used for their antihistamine properties, are not recommended for use with OMNIPAQUE. Others include MAO inhibitors, tricyclic antidepressants, CNS stimulants, and psychoactive drugs described as analeptics, major tranquilizers, or antipsychotic drugs. While the contributory role of these medications has not been established, the use of such drugs should be based on physician evaluation of potential benefits and potential risks. Physicians have discontinued these agents at least 48 hours before and for at least 24 hours postprocedure.
Care is required in patient management to prevent inadvertent intracranial entry of a large dose or concentrated bolus of the medium. Also, effort should be directed to avoid rapid dispersion of the medium causing inadvertent rise to intracranial levels (eg, by active patient movement). Direct intracisternal or ventricular administration for standard radiography (not CT) is not recommended.
In most reported cases of major motor seizures with nonionic myelographic media, one or more of the following factors were present. Therefore avoid:
- Deviations from recommended procedure or in myelographic management.
- Use in patients with a history of epilepsy.
- Overdosage.
- Intracranial entry of a bolus or premature diffusion of a high concentration of the medium.
- Medication with neuroleptic drugs or phenothiazine antinauseants.
- Failure to maintain elevation of the head during the procedure, on the stretcher, or in bed.
- Excessive and particularly active patient movement or straining.
-
PRECAUTIONS—General
Diagnostic procedures which involve the use of radiopaque diagnostic agents should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the particular procedure to be performed. Appropriate facilities should be available for coping with any complication of the procedure, as well as for emergency treatment of severe reactions to the contrast agent itself. After parenteral administration of a radiopaque agent, competent personnel and emergency facilities should be available for at least 30 to 60 minutes since severe delayed reactions have occurred. (See ADVERSE REACTIONS.)
Preparatory dehydration is dangerous and may contribute to acute renal failure in patients with advanced vascular disease, diabetic patients, and in susceptible nondiabetic patients (often elderly with preexisting renal disease). Dehydration in these patients seems to be enhanced by the osmotic diuretic action of contrast agents. Patients should be well hydrated prior to and following administration of any contrast medium, including iohexol.
The possibility of a reaction, including serious, life-threatening, fatal, anaphylactoid, cardiovascular or central nervous system reactions, should always be considered (see ADVERSE REACTIONS). Therefore, it is of utmost importance that a course of action be carefully planned in advance for the immediate treatment of serious reactions, and that adequate and appropriate facilities and personnel be readily available in case of any reaction.
The possibility of an idiosyncratic reaction in susceptible patients should always be considered (see ADVERSE REACTIONS). The susceptible population includes, but is not limited to, patients with a history of a previous reaction to contrast media, patients with a known sensitivity to iodine per se, and patients with a known clinical hypersensitivity: bronchial asthma, hay fever, and food allergies.
The occurrence of severe idiosyncratic reactions has prompted the use of several pretesting methods. However, pretesting cannot be relied upon to predict severe reactions and may itself be hazardous for the patient. It is suggested that a thorough medical history with emphasis on allergy and hypersensitivity, prior to the injection of any contrast media, may be more accurate than pretesting in predicting potential adverse reactions.
A positive history of allergies or hypersensitivity does not arbitrarily contraindicate the use of a contrast agent where a diagnostic procedure is thought essential, but caution should be exercised (see ADVERSE REACTIONS). Premedication with antihistamines or corticosteroids these patients should be considered. Pretreatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
In patients with severe renal insufficiency or failure, compensatory biliary excretion of the drug is anticipated to occur, with a slow clearance into the bile. Patients with hepatorenal insufficiency should not be examined unless the possibility of benefit clearly outweighs the additional risk.
Administration of contrast media should be performed by qualified personnel familiar with the procedure and appropriate patient management (see PATIENT MANAGEMENT). Sterile technique must be used with any spinal puncture.
If nondisposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration. If particulate matter or discoloration is present, do not use.
Repeat Procedures
If in the clinical judgment of the physician sequential or repeat examinations are required, a suitable interval of time between administrations should be observed to allow for normal clearance of the drug from the body (see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY).
Information for Patients (or if applicable, parents of pediatric patients)
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform your physician if you are pregnant (see CLINICAL PHARMACOLOGY).
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease or known thyroid disorder (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food, or if you had any reactions to previous injections of dyes used for x-ray procedures (see PRECAUTIONS—General).
- Inform your physician about any other medications you are currently taking, including non-prescription drugs, before you are administered this drug.
Drug Interactions
Drugs which lower seizure threshold, especially phenothiazine derivatives including those used for their antihistaminic or antinauseant properties, are not recommended for use with OMNIPAQUE. Others include monoamine oxidase (MAO) inhibitors, tricyclic antidepressants, CNS stimulants, psychoactive drugs described as analeptics, major tranquilizers, or antipsychotic drugs. Such medications should be discontinued at least 48 hours before myelography, should not be used for the control of nausea or vomiting during or after myelography, and should not be resumed for at least 24 hours postprocedure. In nonelective procedures in patients on these drugs, consider prophylactic use of anticonvulsants.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed with OMNIPAQUE to evaluate carcinogenic potential. OMNIPAQUE was not genotoxic in a series of studies, including the Ames test, the mouse lymphoma TK locus forward mutation assay, and a mouse micronucleus assay. OMNIPAQUE did not impair the fertility of male or female rats when administered at dosages up to 4 g Iodine/kg (2.3 times the maximum recommended dose for a 50 kg human, or approximately 0.4 times the maximum recommended dose for a 50 kg human following normalization of the data to body surface area estimates.)
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies performed in rats and rabbits at dosages up to 4 g Iodine/kg and 2.5 g Iodine/kg, respectively [2.3 and 1.4 times the maximum recommended dose for a 50 kg human, or approximately 0.4 (rat) and 0.5 (rabbit) times the maximum recommended dose for a 50 kg human following normalization of the data to body surface area estimates] have not revealed evidence of impaired fertility or harm to the fetus due to OMNIPAQUE. Adequate and well-controlled studies in pregnant women have not been conducted. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known to what extent iohexol is excreted in human milk. However, many injectable contrast agents are excreted unchanged in human milk. Although it has not been established that serious adverse reactions occur in nursing infants, caution should be exercised when intravascular contrast media are administered to nursing women. Bottle feedings may be substituted for breast feedings for 24 hours following administration of OMNIPAQUE.
Pediatric Use
Pediatric patients at higher risk of experiencing adverse events during contrast medium administration may include those having asthma, a sensitivity to medication and/or allergens, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age.
-
ADVERSE REACTIONS—Intrathecal
The most frequently reported adverse reactions with OMNIPAQUE are headache, backache, neckache and stiffness, nausea, and vomiting. These reactions usually occur 1 to 10 hours after injection, and almost all occur within 24 hours. They usually last for a few hours, and usually disappear within 24 hours. Severe headaches persisting for days have been reported. Headache is often accompanied by nausea and vomiting and tends to be more frequent and persistent in patients not optimally hydrated.
Transient alterations in vital signs may occur and their significance must be assessed on an individual basis. Those reactions reported in clinical studies with OMNIPAQUE are listed below in decreasing order of occurrence, based on clinical studies of 1531 patients.
Headaches: The most frequently occurring adverse reaction following myelography has been headache, with an incidence of approximately 18%. Headache may be caused by either a direct effect of the contrast medium or by CSF leakage at the dural puncture site. However, in managing the patient , it is considered more important to minimize intracranial entry of contrast medium by postural management than attempting to control possible CSF leakage (see PATIENT MANAGEMENT).
Pain: Backache, neckache and stiffness, and neuralgia occurred following injection with an incidence of about 8%.
Nausea and Vomiting: Nausea was reported with an incidence of about 6%, and vomiting about 3% (see PATIENT MANAGEMENT). Maintaining normal hydration is very important. The use of phenothiazine antinauseants is not recommended. (See WARNINGS—General.) Reassurance to the patient that the nausea will clear usually is all that is required.
Dizziness: Transient dizziness was reported in about 2% of the patients.
Other Reactions: Other reactions occurring with an individual incidence of less than 0.1% included: feeling of heaviness, hypotension, hypertonia, sensation of heat, sweating, vertigo, loss of appetite, drowsiness, hypertension, photophobia, tinnitus, neuralgia, paresthesia, difficulty in micturition, and neurological changes.
General Adverse Reactions to Contrast Media
Physicians should remain alert for the occurrence of adverse effects in addition to those discussed above, particularly the following reactions which have been reported in the literature for nonionic, water-soluble myelographic media including iohexol. These have included, but are not limited to, convulsion, aseptic and bacterial meningitis, and CNS and other neurological disturbances.
An aseptic meningitis syndrome has been reported (in less than 0.01%). It was usually preceded by pronounced headaches, nausea and vomiting. Onset usually occurred about 12 to 18 hours postprocedure. Prominent features were meningismus, fever, sometimes with oculomotor signs and mental confusion. Lumbar puncture revealed a high white cell count, high protein content often with a low glucose level and with absence of organisms. The condition usually started to clear spontaneously about 10 hours after onset, with complete recovery over 2 to 3 days.
Allergy or Idiosyncrasy: Chills, fever, profuse diaphoresis, pruritus, urticaria, nasal congestion, dyspnea, anaphylactic reactions, anaphylactic shock, and a case of Guillain-Barré syndrome.
CNS Irritation: Transient perceptual aberrations such as hallucinations, depersonalization, amnesia, hostility, amblyopia, diplopia, photophobia, psychosis, insomnia, anxiety, depression, hyperesthesia, visual or auditory or speech disturbances, confusion and disorientation. In addition, malaise, weakness, convulsion, EEG changes, meningism, hyperreflexia or areflexia, hypertonia or flaccidity, hemiplegia, paralysis, quadriplegia, restlessness, tremor, echoacousia, echolalia, asterixis, cerebral hemorrhage, and dysphasia have occurred.
Profound mental disturbances have also been reported. They consisted of various forms and degrees of aphasia, mental confusion, or disorientation. The onset is usually at 8 to 10 hours and lasts for about 24 hours, without aftereffects. Apprehension, agitation, or progressive withdrawal in several instances to the point of somnolence, stupor, and coma have been reported, as well as transitory hearing loss or other auditory symptoms and visual disturbances, including unilateral or bilateral loss of vision which may last for hours. In one case, persistent cortical loss of vision has been reported in association with convulsions. Ventricular block has been reported; amnesia of varying degrees may be present for the reaction event.
Persistent though transitory weakness in the leg or ocular muscles has been reported.
Peripheral neuropathies have been reported. They include sensory and/or motor or nerve root disturbances, myelitis, persistent leg muscle pain or weakness, 6th nerve palsy, or cauda equina syndrome. Muscle cramps, fasciculation or myoclonia, spinal convulsion, or spasticity responded promptly to a small intravenous dose of diazepam. In general, the reactions which are known to occur upon parenteral administration of iodinated contrast agents are possible with any nonionic agent. Severe, life-threatening, fatal anaphylactic shock has been reported.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physicochemical properties of the contrast media, the dose, and speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate, and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
The reported incidence of adverse reactions to contrast media in patients with a history of allergy is twice that of the general population. Patients with a history of previous reactions to a contrast medium are three times more susceptible than other patients. However, sensitivity to contrast media does not appear to increase with repeated examinations. Most adverse reactions to injectable contrast media appear within 1 to 3 minutes after the start of injection, but delayed reactions may occur.
-
OVERDOSAGE
Clinical consequences of overdosage with OMNIPAQUE have not been reported. However, based on experience with other nonionic myelographic media, physicians should be alert to a potential increase in frequency and severity of CNS-mediated reactions. Even use of a recommended dose can produce effects tantamount to overdosage, if incorrect management of the patient during or immediately following the procedure permits inadvertent early intracranial entry of a large portion of the medium.
The intracisternal LD 50 value of OMNIPAQUE (in grams of iodine per kilogram body weight) is greater than 2 in mice.
-
DOSAGE AND ADMINISTRATION — Intrathecal
The volume and concentration of OMNIPAQUE 300 to be administered will depend on the degree and extent of contrast required in the area(s) under examination and on the equipment and technique employed.
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is recommended for the examination of the lumbar, thoracic, and cervical regions in adults by lumbar or direct cervical injection and is slightly hypertonic to CSF.
A total dose of 3060 mg iodine or a concentration of 300 mg Iodine/mL should not be exceeded in adults in a single myelographic examination. This is based on clinical trial evaluation to date. As in all diagnostic procedures, the minimum volume and dose to produce adequate visualization should be used. Most procedures do not require either maximum dose or concentration.
Anesthesia is not necessary. Premedication sedatives or tranquilizers are usually not needed (see PRECAUTIONS). Patients should be well hydrated prior to and following contrast administration. Seizure-prone patients should be maintained on anticonvulsant medication. Many radiopaque contrast agents are incompatible in vitro with some antihistamines and many other drugs; therefore, concurrent drugs should not be physically admixed with contrast agents.
Rate of Injection
To avoid excessive mixing with CSF and consequent dilution of contrast, injection should be made slowly over 1 to 2 minutes.
Depending on the estimated volume of contrast medium which may be required for the procedure a small amount of CSF may be removed to minimize distention of the subarachnoid spaces.
The lumbar or cervical puncture needle may be removed immediately following injection since it is not necessary to remove OMNIPAQUE after injection into the subarachnoid space.
Adults
The usual recommended total doses for use in lumbar, thoracic, cervical, and total columnar myelography in adults are 1.2 g Iodine to 3 g Iodine as follows:
Procedure Formulations Concentration
(mg Iodine/mL)Volume
(mL)Dose
(g Iodine)Thoracic Myelography
(via lumbar injection)OMNIPAQUE 300 300 6-10 1.8-3.0 Cervical Myelography
(via lumbar injection)OMNIPAQUE 300 300 6-10 1.8-3.0 Cervical Myelography
(via C1-2 injection)OMNIPAQUE 300 300 4-10 1.2-3.0 Total Columnar Myelography
(via lumbar injection)OMNIPAQUE 300 300 6-10 1.8-3.0 Refer to DIRECTIONS FOR PROPER USE OF OMNIPAQUE PHARMACY BULK PACKAGE section for instructions.
Parenteral products should be inspected visually for particulate matter or discoloration prior to administration. If particulate matter or discoloration is present, do not use.
Repeat Procedures
If in the clinical judgment of the physician sequential or repeat examinations are required, a suitable interval of time between administrations should be observed to allow for normal clearance of the drug from the body. An interval of at least 48 hours should be allowed before repeat examination; however, whenever possible, 5 to 7 days is recommended.
-
PATIENT MANAGEMENT—Intrathecal
Suggestions for Usual Patient Management
Good patient management should be exercised at all times to minimize the potential for procedurally related complications.
Preprocedure
- Discontinuance of neuroleptic drugs (including phenothiazines, eg, chlorpromazine, prochlorperazine, and promethazine) at least 48 hours beforehand should be considered.
- Maintain normal diet up to 2 hours before procedure.
- Ensure hydration—fluids up to procedure.
During Procedure
- Use minimum dose and concentration required for satisfactory contrast (see DOSAGE AND ADMINISTRATION).
- In all positioning techniques keep the patient's head elevated above highest level of spine.
- Do not lower head of table more than 15° in moving contrast medium cranially.
- In patients with excessive lordosis, consider lateral position for injection and movement of the medium cephalad.
- Inject slowly (over 1 to 2 minutes) to avoid excessive mixing.
- To maintain as a bolus, move medium to distal area very slowly. Use fluoroscopic monitoring.
- Avoid intracranial entry of a bolus.
- Avoid early and high cephalad dispersion of the medium.
- Avoid abrupt or active patient movement to minimize excessive mixing of medium with CSF. Instruct patient to remain passive. Move patient slowly and only as necessary.
Postprocedure
- Raise head of stretcher to at least 30° before moving patient onto it.
- Movement onto and off the stretcher should be done slowly with the patient completely passive, maintaining head-up position.
- Before moving patient onto bed, raise head of bed 30° to 45°.
- Advise patient to remain still in bed, in a sitting or semisitting position, especially in the first few hours.
- Maintain close observation for at least 12 hours after myelogram.
- Obtain visitors' cooperation in keeping the patient quiet and in head-up position, especially in first few hours.
- Encourage oral fluids. Diet as tolerated.
- If nausea or vomiting occurs, do not use phenothiazine antinauseants. Persistent nausea and vomiting will result in dehydration. Therefore, prompt consideration of replacement by intravenous fluids is recommended.
Alternative Postprocedure Method
- Recent evidence with nonionic, water-soluble contrast media suggests that maintaining the patient postmyelography in an upright position (via wheelchair or ambulation) may help minimize adverse effects. The upright position may help to delay upward dispersion of the medium and to maximize the spinal arachnoid absorption.
- SECTION II
-
CLINICAL PHARMACOLOGY—Intravascular
Following intravascular injection, iohexol is distributed in the extracellular fluid compartment and is excreted unchanged by glomerular filtration. It will opacify those vessels in the path of flow of the contrast medium permitting radiographic visualization of the internal structures until significant hemodilution occurs.
Approximately 90% or more of the injected dose is excreted within the first 24 hours, with the peak urine concentrations occurring in the first hour after administration. Plasma and urine iohexol levels indicate that the iohexol body clearance is due primarily to renal clearance. An increase in the dose from 500 mg Iodine/kg to 1500 mg Iodine/kg does not significantly alter the clearance of the drug. The following pharmacokinetic values were observed following the intravenous administration of iohexol (between 500 mg Iodine/kg to 1500 mg Iodine/kg) to 16 adult human subjects: renal clearance—120 (86-162) mL/min; total body clearance—131 (98-165) mL/min; and volume of distribution—165 (108-219) mL/kg.
Renal accumulation is sufficiently rapid that the period of maximal opacification of the renal passages may begin as early as 1 minute after intravenous injection. Urograms become apparent in about 1 to 3 minutes with optimal contrast occurring between 5 to 15 minutes.
In nephropathic conditions, particularly when excretory capacity has been altered, the rate of excretion may vary unpredictably, and opacification may be delayed after injection. Severe renal impairment may result in a lack of diagnostic opacification of the collecting system and, depending on the degree of renal impairment, prolonged plasma iohexol levels may be anticipated. In these patients, as well as in infants with immature kidneys, the route of excretion through the gallbladder and into the small intestine may increase.
Iohexol displays a low affinity for serum or plasma proteins and is poorly bound to serum albumin. No significant metabolism, deiodination or biotransformation occurs.
OMNIPAQUE probably crosses the placental barrier in humans by simple diffusion. It is not known to what extent iohexol is excreted in human milk.
Animal studies indicate that iohexol does not cross an intact blood-brain barrier to any significant extent following intravascular administration.
OMNIPAQUE enhances computed tomographic imaging through augmentation of radiographic efficiency. The degree of density enhancement is directly related to the iodine content in an administered dose; peak iodine blood levels occur immediately following rapid intravenous injection. Blood levels fall rapidly within 5 to 10 minutes and the vascular compartment half-life is approximately 20 minutes. This can be accounted for by the dilution in the vascular and extravascular fluid compartments which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached in about ten minutes; thereafter, the fall becomes exponential.
The pharmacokinetics of iohexol in both normal and abnormal tissue have been shown to be variable. Contrast enhancement appears to be greatest immediately after bolus administration (15 seconds to 120 seconds). Thus, greatest enhancement may be detected by a series of consecutive two-to-three second scans performed within 30 to 90 seconds after injection (ie, dynamic computed tomographic imaging). Utilization of a continuous scanning technique (ie, dynamic CT scanning) may improve enhancement and diagnostic assessment of tumor and other lesions such as abscess, occasionally revealing unsuspected or more extensive disease. For example, a cyst may be distinguished from a vascularized solid lesion when precontrast and enhanced scans are compared; the nonperfused mass shows unchanged x-ray absorption (CT number). A vascularized lesion is characterized by an increase in CT number in the few minutes after a bolus of intravascular contrast agent; it may be malignant, benign, or normal tissue, but would probably not be a cyst, hematoma, or other nonvascular lesion.
Because unenhanced scanning may provide adequate diagnostic information in the individual patient, the decision to employ contrast enhancement, which may be associated with risk and increased radiation exposure, should be based upon a careful evaluation of clinical, other radiological, and unenhanced CT findings.
-
INDICATIONS AND USAGE GENERAL—Intravascular
OMNIPAQUE 350 is indicated in adults for angiocardiography (ventriculography, selective coronary arteriography), aortography including studies of the aortic root, aortic arch, ascending aorta, abdominal aorta and its branches, intravenous digital subtraction angiography of the head, neck, abdominal, renal and peripheral vessels, peripheral arteriography, and excretory urography.
OMNIPAQUE 350 is indicated in pediatric patients for angiocardiography (ventriculography, pulmonary arteriography, and venography; studies of the collateral arteries and aortography, including the aortic root, aortic arch, ascending and descending aorta). OMNIPAQUE 300 is indicated in adults for aortography including studies of the aortic arch, abdominal aorta and its branches, cerebral arteriography, peripheral venography (phlebography), and excretory urography.
OMNIPAQUE 300 is indicated in pediatric patients for angiocardiography (ventriculography), excretory urography.
- CONTRAINDICATIONS
-
WARNINGS—General
Nonionic iodinated contrast media inhibit blood coagulation, in vitro, less than ionic contrast media. Clotting has been reported when blood remains in contact with syringes containing nonionic contrast media.
Serious, fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state, and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
OMNIPAQUE should be used with extreme care in patients with severe functional disturbances of the liver and kidneys, severe thyrotoxicosis, or myelomatosis. Diabetics with a serum creatinine level above 3 mg/dL should not be examined unless the possible benefits of the examination clearly outweigh the additional risk. OMNIPAQUE is not recommended for use in patients with anuria.
Radiopaque contrast agents are potentially hazardous in patients with multiple myeloma or other paraproteinemia, particularly in those with therapeutically resistant anuria. Although neither the contrast agent nor dehydration has separately proven to be the cause of anuria in myeloma, it has been speculated that the combination of both may be causative factors. The risk in myelomatous patients is not a contraindication; however, special precautions are necessary. Partial dehydration in the preparation of these patients prior to injection is not recommended since this may predispose the patient to precipitation of the myeloma protein in the renal tubules. No form of therapy, including dialysis, has been successful in reversing the effect. Myeloma, which occurs most commonly in persons over age 40, should be considered before instituting intravascular administration of contrast agents.
Ionic contrast media, when injected intravenously or intra-arterially, may promote sickling in individuals who are homozygous for sickle cell disease.
Administration of radiopaque materials to patients known or suspected of having pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The patient's blood pressure should be assessed throughout the procedure and measures for the treatment of hypertensive crisis should be readily available. Reports of thyroid storm following the use of iodinated contrast media in patients with hyperthyroidism or with an autonomously functioning thyroid nodule suggest that this additional risk be evaluated in such patients before use of any contrast medium.
Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age
Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast media (ICM) in pediatric patients 0 to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at the greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular OMNIPAQUE administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering OMNIPAQUE to patients with a history of a severe cutaneous adverse reaction to OMNIPAQUE.
Urography should be performed with caution in patients with severely impaired renal function and patients with combined renal and hepatic disease.
-
PRECAUTIONS—General
Diagnostic procedures which involve the use of radiopaque diagnostic agents should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the particular procedure to be performed. Appropriate facilities should be available for coping with any complication of the procedure, as well as for emergency treatment of severe reactions to the contrast agent itself. After parenteral administration of a radiopaque agent, competent personnel and emergency facilities should be available for at least 30 to 60 minutes since severe delayed reactions have occurred (see ADVERSE REACTIONS: Intravascular—General).
Preparatory dehydration is dangerous and may contribute to acute renal failure in patients with advanced vascular disease, diabetic patients, and in susceptible nondiabetic patients (often elderly with preexisting renal disease), infants and small pediatric patients. Dehydration in these patients seems to be enhanced by the osmotic diuretic action of urographic agents. It is believed that overnight fluid restriction prior to excretory urography generally does not provide better visualization in normal patients. Patients should be well hydrated prior to and following administration of any contrast medium, including iohexol.
Acute renal failure has been reported in diabetic patients with diabetic nephropathy and in susceptible non-diabetic patients (often elderly with preexisting renal disease) following excretory urography. Therefore, careful consideration of the potential risks should be given before performing this radiographic procedure in these patients.
Immediately following surgery, excretory urography should be used with caution in renal transplant recipients.
The possibility of a reaction, including serious, life-threatening, fatal, anaphylactoid or cardiovascular reactions should always be considered (see ADVERSE REACTIONS: Intravascular—General). It is of utmost importance that a course of action be carefully planned in advance for immediate treatment of serious reactions, and that adequate and appropriate personnel be readily available in case of any reaction.
The possibility of an idiosyncratic reaction in susceptible patients should always be considered (see ADVERSE REACTIONS: Intravascular—General). The susceptible population includes, but is not limited to, patients with a history of a previous reaction to contrast media, patients with a known sensitivity to iodine per se, and patients with a known clinical hypersensitivity: bronchial asthma, hay fever, and food allergies.
The occurrence of severe idiosyncratic reactions has prompted the use of several pretesting methods. However, pretesting cannot be relied upon to predict severe reactions and may itself be hazardous for the patient. It is suggested that a thorough medical history with emphasis on allergy and hypersensitivity, prior to the injection of any contrast media, may be more accurate than pretesting in predicting potential adverse reactions.
A positive history of allergies or hypersensitivity does not arbitrarily contraindicate the use of a contrast agent where a diagnostic procedure is thought essential, but caution should be exercised (see ADVERSE REACTIONS: Intravascular—General). Premedication with antihistamines or corticosteroids in these patients should be considered. Recent reports indicate that such pretreatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
Even though the osmolality of OMNIPAQUE is low compared to diatrizoate or iothalamate- based ionic agents of comparable iodine concentration, the potential transitory increase in the circulatory osmotic load in patients with congestive heart failure requires caution during injection. These patients should be observed for several hours following the procedure to detect delayed hemodynamic disturbances.
General anesthesia may be indicated in the performance of some procedures in selected adult patients; however, a higher incidence of adverse reactions has been reported in these patients, and may be attributable to the inability of the patient to identify untoward symptoms, or to the hypotensive effect of anesthesia which can reduce cardiac output and increase the duration of exposure to the contrast agent.
Angiography should be avoided whenever possible in patients with homocystinuria, because of the risk of inducing thrombosis and embolism.
In angiographic procedures, the possibility of dislodging plaques or damaging or perforating the vessel wall should be borne in mind during the catheter manipulations and contrast medium injection. Test injections to ensure proper catheter placement are recommended. Selective coronary arteriography should be performed only in those patients in whom the expected benefits outweigh the potential risk. The inherent risks of angiocardiography in patients with chronic pulmonary emphysema must be weighed against the necessity for performing this procedure.
If nondisposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration. If particulate matter or discoloration is present, do not use.
Information for Patients
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform your physician if you are pregnant (see CLINICAL PHARMACOLOGY—Intravascular).
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease, or known thyroid disorder (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food, or if you had any reactions to previous injections of dyes used for x-ray procedures (see PRECAUTIONS—General).
- Inform your physician about any other medications you are currently taking, including non-prescription drugs, before you are administered this drug.
- Advise patients to inform their physician if they develop a rash after receiving OMNIPAQUE.
- Advise parents/caregivers about the risk of developing thyroid dysfunction after OMNIPAQUE administration. Advise parents/caregivers about when to seek medical care for their child to monitor for thyroid function. [see WARNINGS]
Drug/Laboratory Test Interaction
If iodine-containing isotopes are to be administered for the diagnosis of thyroid disease, the iodine-binding capacity of thyroid tissue may be reduced for up to 2 weeks after contrast medium administration. Thyroid function tests which do not depend on iodine estimation, eg, T 3 resin uptake or direct thyroxine assays, are not affected.
Many radiopaque contrast agents are incompatible in vitro with some antihistamines and many other drugs; therefore, no other pharmaceuticals should be admixed with contrast agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed with OMNIPAQUE to evaluate carcinogenic potential. OMNIPAQUE was not genotoxic in a series of studies, including the Ames test, the mouse lymphoma TK locus forward mutation assay, and a mouse micronucleus assay. OMNIPAQUE did not impair the fertility of male or female rats when administered at dosages up to 4 g Iodine/kg (2.3 times the maximum recommended dose for a 50 kg human, or approximately 0.4 times the maximum recommended dose for a 50 kg human following normalization of the data to body surface area estimates.)
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies performed in rats and rabbits at dosages up to 4 g Iodine/kg and 2.5 g Iodine/kg, respectively [2.3 and 1.4 times the maximum recommended dose for a 50 kg human, or approximately 0.4 (rat) and 0.5 (rabbit) times the maximum recommended dose for a 50 kg human following normalization of the data to body surface area estimates] have not revealed evidence of impaired fertility or harm to the fetus due to OMNIPAQUE. Adequate and well-controlled studies in pregnant women have not been conducted. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known to what extent iohexol is excreted in human milk. However, many injectable contrast agents are excreted unchanged in human milk. Although it has not been established that serious adverse reactions occur in nursing infants, caution should be exercised when intravascular contrast media are administered to nursing women. Bottle feedings may be substituted for breast feedings for 24 hours following administration of OMNIPAQUE.
Pediatric Use
Pediatric patients at higher risk of experiencing adverse events during contrast medium administration may include those having asthma, a sensitivity to medication and/or allergens, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age. Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates. Some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 to 3 years of age based on underlying risk factors, especially in term and preterm neonates (see WARNINGS and ADVERSE REACTIONS).
-
ADVERSE REACTIONS: Intravascular—General
Serious, life-threatening and fatal reactions, mostly of cardiovascular origin, have been associated with the administration of iodine-containing contrast media, including OMNIPAQUE. The injection of contrast media is frequently associated with the sensation of warmth and pain, especially in peripheral angiography; pain and warmth are less frequent and less severe with OMNIPAQUE than with many contrast media. Cardiovascular System: Arrhythmias including PVCs and PACs (2%), angina/chest pain (1%), and hypotension (0.7%). Others including cardiac failure, asystole, bradycardia, tachycardia, and vasovagal reaction were reported with an individual incidence of 0.3% or less.
Nervous System: Vertigo (including dizziness and lightheadedness) (0.5%), pain (3%), vision abnormalities (including blurred vision and photomas) (2%), headache (2%), and taste perversion (1%). Others including anxiety, fever, motor and speech dysfunction, convulsion, paresthesia, somnolence, stiff neck, hemiparesis, syncope, shivering, transient ischemic attack, cerebral infarction, and nystagmus were reported, with an individual incidence of 0.3% or less.
Respiratory System: Dyspnea, rhinitis, coughing, and laryngitis, with an individual incidence of 0.2% or less.
Gastrointestinal System: Nausea (2%) and vomiting (0.7%). Others including diarrhea, dyspepsia, cramp, and dry mouth were reported, with an individual incidence of less than 0.1%.
Skin and Appendages: Urticaria (0.3%), purpura (0.1%), abscess (0.1%), and pruritus (0.1%). Individual adverse reactions which occurred to a significantly greater extent for a specific procedure are listed under that indication.
Pediatrics
In controlled clinical trials involving 391 patients for pediatric angiocardiography and urography, adverse reactions following the use of OMNIPAQUE 300 and OMNIPAQUE 350 were generally not more frequent than with adults.
Cardiovascular System: Ventricular tachycardia (0.5%), 2:1 heart block (0.5%), hypertension (0.3%), and anemia (0.3%).
Nervous System: Pain (0.8%), fever (0.5%), taste abnormality (0.5%), and convulsion (0.3%).
Respiratory System: Congestion (0.3%) and apnea (0.3%).
Gastrointestinal System: Nausea (1%), hypoglycemia (0.3%), and vomiting (2%).
Skin and Appendages: Rash (0.3%).
General Adverse Reactions to Contrast Media
Physicians should remain alert for the occurrence of adverse effects in addition to those discussed above.
The following reactions have been reported after administration of - intravascular iodinated contrast media. Reactions due to technique: hematomas and ecchymoses. Hemodynamic reactions: vein cramp and thrombophlebitis following intravenous injection. Cardiovascular reactions: cardiac arrhythmias, reflex tachycardia, chest pain, cyanosis, hypertension, hypotension, peripheral vasodilatation, shock, and cardiac arrest. Renal reactions: transient proteinuria, oliguria or anuria. Endocrine reactions: hyperthyroidism, hypothyroidism .Skin and Subcutaneous Tissue Disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria, skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)]. Allergic reactions: asthmatic attacks, nasal and conjunctival symptoms, dermal reactions such as urticaria with or without pruritus, as well as pleomorphic rashes, sneezing and lacrimation, anaphylactic reactions and anaphylactic shock. Fatalities have occurred, due to this or unknown causes. S igns and symptoms related to the respiratory system: pulmonary or laryngeal edema, bronchospasm, dyspnea; or to the nervous system: restlessness, tremors, convulsions. Other reactions: flushing, pain, warmth, metallic taste, nausea, vomiting, anxiety, headache, confusion, pallor, weakness, sweating, localized areas of edema, especially facial cramps, neutropenia, and dizziness. Immediate or delayed rigors can occur, sometimes accompanied by hyperpyrexia. Infrequently, "iodism" (salivary gland swelling) from organic iodinated compounds appears two days after exposure and subsides by the sixth day.
In general, the reactions which are known to occur upon parenteral administration of iodinated contrast agents are possible with any nonionic agent. Severe, life-threatening, fatal anaphylactoid shock has been reported. Reported incidences of death range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 (0.01 percent). Most deaths occur during injection or 5 to 10 minutes later; the main feature being cardiac arrest with cardiovascular disease as the main aggravating factor.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physicochemical properties of the contrast media, the dose, and speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate, and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
The reported incidence of adverse reactions to contrast media in patients with a history of allergy are twice that of the general population. Patients with a history of previous reactions to a contrast medium are three times more susceptible than other patients. However, sensitivity to contrast media does not appear to increase with repeated examinations.
Most adverse reactions to injectable contrast media appear within 1 to 3 minutes after the start of injection, but delayed reactions may occur.
Regardless of the contrast agent employed, the overall estimated incidence of serious adverse reactions is higher with angiocardiography than with other procedures. Cardiac decompensation, serious arrhythmias, angina pectoris, or myocardial ischemia or infarction may occur during angiocardiography and left ventriculography. Immediately following intravascular injection of contrast medium, a transient sensation of mild warmth is not unusual. Warmth is less frequent with OMNIPAQUE than with ionic media. (see ADVERSE REACTIONS: Intravascular—General)
-
OVERDOSAGE
Overdosage may occur. The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular systems. The symptoms included: cyanosis, bradycardia, acidosis, pulmonary hemorrhage, convulsions, coma, and cardiac arrest. Treatment of an overdosage is directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
The intravenous LD 50 values of OMNIPAQUE (in grams of iodine per kilogram body weight) are 24.2 in mice and 15.0 in rats.
-
DOSAGE AND ADMINISTRATION — General
As with all radiopaque contrast agents, the lowest dose of OMNIPAQUE necessary to obtain adequate visualization should be used. A lower dose may reduce the possibility of an adverse reaction. Most procedures do not require use of either the maximum volume or the highest concentration of OMNIPAQUE. The combination of volume and concentration of OMNIPAQUE to be used should be carefully individualized accounting for factors such as age, body weight, size of the vessel and the rate of blood flow within the vessel. Other factors such as anticipated pathology, degree and extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed should be considered.
Sterile technique must be used in all vascular injections involving contrast media.
Refer to DIRECTIONS FOR PROPER USE OF OMNIPAQUE PHARMACY BULK PACKAGE section for instructions.
If nondisposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents.
It may be desirable that solutions of radiopaque diagnostic agents be used at body temperature when injected.
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Solutions of OMNIPAQUE should be used only if clear and within the normal colorless to pale yellow range. If particulate matter or discoloration is present, do not use.
-
INDIVIDUAL INDICATIONS AND USAGE
ANGIOCARDIOGRAPHY
Pharmacology—Hemodynamic Changes
OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in adults for angiocardiography (ventriculography, aortic root injections, and selective coronary arteriography). OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in pediatric patients for angiocardiography (ventriculography, pulmonary arteriography, and venography, and studies of the collateral arteries).
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in pediatric patients pediatric patients for angiocardiography (ventriculography).
After both ventricular and coronary injection, decreases in systolic pressure were less pronounced and returned to baseline values earlier with OMNIPAQUE 350 than with diatrizoate meglumine and diatrizoate sodium injection.
OMNIPAQUE 350 produced less Q-T interval prolongation than seen with diatrizoate meglumine and diatrizoate sodium injection.
In pediatric patients, after injection of all sites, but particularly following ventricular and pulmonary artery injections, decreases in both systolic and diastolic intravascular pressure were significantly less pronounced with OMNIPAQUE 350 than with diatrizoate meglumine and diatrizoate sodium injection. In pediatric patients OMNIPAQUE 350 produced significantly less shortening of the R-R interval than seen with diatrizoate meglumine and diatrizoate sodium injection.
If repeat injections are made in rapid succession, all these changes are likely to be more pronounced. (See DOSAGE AND ADMINISTRATION.)
Precautions
During administration of large doses of OMNIPAQUE 350, continuous monitoring of vital signs is desirable. Caution is advised in the administration of large volumes to patients with incipient heart failure because of the possibility of aggravating the preexisting condition. Hypotension should be corrected promptly since it may induce serious arrhythmias. Special care regarding dosage should be observed in patients with right ventricular failure, pulmonary hypertension, or stenotic pulmonary vascular beds because of the hemodynamic changes which may occur after injection into the right heart outflow tract. (See PRECAUTIONS—General.)
Pediatric patients at higher risk of experiencing adverse events during contrast medium administration may include those having asthma, a sensitivity to medication and/or allergens, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age.
Adverse Reactions
Cardiovascular system reactions in angiocardiography included angina (8%), hypotension (2.5%), bradycardia (1%), and tachycardia (1%). (See ADVERSE REACTIONS: Intravascular—General.)
Dosage and Administration
The individual dose or volume is determined by the size of the structure to be visualized, the anticipated degree of hemodilution, and valvular competence. Weight is a minor consideration in adults, but must be considered in infants and young pediatric patients. The volume of each individual injection is a more important consideration than the total dosage used. When large individual volumes are administered, as in ventriculography and aortography, it has been suggested that several minutes be permitted to elapse between each injection to allow for subsidence of possible hemodynamic disturbances.
The recommended single injection volume of OMNIPAQUE 350 for angiocardiographic procedures in adults and the recommended single injection volumes of OMNIPAQUE 350 and OMNIPAQUE 300 for angiographic procedures in pediatric patients are as follows:
Ventriculography
Adults
The usual adult volume for a single injection is 40 mL with a range of 30 mL to 60 mL. This may be repeated as necessary. When combined with selective coronary arteriography, the total administered volume should not exceed 250 mL (87.5 g Iodine).
Pediatrics
The usual single injection dose of OMNIPAQUE 350 is 1.25 mL/kg of body weight with a range of 1 mL/kg to 1.5 mL/kg. For OMNIPAQUE 300 the usual single injection dose is 1.75 mL/kg with a range of 1.5 mL/kg to 2.0 mL/kg. When multiple injections are given, the total administered dose should not exceed 5 mL/kg up to a total volume of 250 mL of OMNIPAQUE 350 or up to a total volume of 291 mL of OMNIPAQUE 300.
Selective Coronary Arteriography
The usual adult volume for right or left coronary arteriography is 5 mL (range 3 mL to 14 mL) per injection.
Aortic Root and Arch Study When Used Alone
The usual adult single injection volume is 50 mL, with a range of 20 mL to 75 mL.
Combined Angiocardiographic Procedures
Multiple Procedures
Adults
The visualization of multiple vascular systems and target organs is possible during a single radiographic examination of the patient.
Large doses of OMNIPAQUE 350 were well tolerated in angiographic procedures requiring multiple injections.
The maximum total volume for multiple procedures should not exceed 250 mL of 350 mg Iodine/mL (87.5 g Iodine).
Pediatrics
Visualization of multiple vascular systems and target organs is possible during a single radiographic examination of the patient.
The maximum total dose for multiple injection procedures should not exceed 5 mL/kg up to a total volume of 250 mL of OMNIPAQUE 350 or 6 mL/kg up to a total volume of 291 mL of OMNIPAQUE 300.
AORTOGRAPHY AND SELECTIVE VISCERAL ARTERIOGRAPHY
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL and OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL are indicated in adults for use in aortography and selective visceral arteriography including studies of the aortic arch, ascending aorta, and abdominal aorta and its branches (celiac, mesenteric, renal, hepatic and splenic arteries).
OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in pediatric patients for use in aortography including studies of the aortic root, aortic arch, ascending and descending aorta.
Precautions
Under conditions of slowed aortic circulation there is an increased likelihood for aortography to cause muscle spasm. Occasional serious neurologic complications, including paraplegia, have also been reported in patients with aortoiliac obstruction, femoral artery obstruction, abdominal compression, hypotension, hypertension, spinal anesthesia, and injection of vasopressors to increase contrast. In these patients the concentration, volume and number of repeat injections of the medium should be maintained at a minimum with appropriate intervals between injections. The position of the patient and catheter tip should be carefully monitored.
Entry of a large aortic dose into the renal artery may cause, even in the absence of symptoms, albuminuria, hematuria, and an elevated creatinine and urea nitrogen. Rapid and complete return of function usually follows. (See PRECAUTIONS—General.)
Adverse Reactions
See ADVERSE REACTIONS: Intravascular—General, and ADVERSE REACTIONS ANGIOCARDIOGRAPHY.
Dosage and Administration
Adults
The usual adult volume as a single injection is 50 mL to 80 mL for the aorta, 30 mL to 60 mL for major branches including celiac and mesenteric arteries, and 5 mL to 15 mL for renal arteries. Repeated injections may be performed if indicated, but the total volume should not exceed 291 mL of OMNIPAQUE 300 or 250 mL of OMNIPAQUE 350 (87.5 g Iodine).
CEREBRAL ARTERIOGRAPHY
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in adults for use in cerebral arteriography.
The degree of pain and flushing as the result of the use of OMNIPAQUE 300 in cerebral arteriography is less than that seen with comparable injections of many contrast media.
In cerebral arteriography, patients should be appropriately prepared consistent with existing or suspected disease states.
Precautions
Cerebral arteriography should be undertaken with extreme care with special caution in elderly patients, patients in poor clinical condition, advanced arteriosclerosis, severe arterial hypertension, recent cerebral embolism or thrombosis, and cardiac decompensation.
Since the contrast medium is given by rapid injection, the patient should be monitored for possible untoward reactions. (See PRECAUTIONS—General.)
Adverse Reactions
Cerebral arteriography with water-soluble contrast media has been associated with temporary neurologic complications including seizures, drowsiness, transient paresis, and mild disturbances in vision such as photomas of 1-second or less duration.
Central nervous system reactions in cerebral arteriography included photomas (15%), headache (5.5%), and pain (4.5%). (See ADVERSE REACTIONS: Intravascular—General.)
DIGITAL SUBTRACTION ANGIOGRAPHY
Intravenous Administration
OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in adults for use in intravenous digital subtraction angiography (I.V.DSA) of the vessels of the head, neck, and abdominal, renal and peripheral vessels.
Arteriograms of diagnostic quality can be obtained following the intravenous administration of contrast media employing digital subtraction and computer imaging enhancement techniques. The intravenous route of administration using these techniques has the advantage of being less invasive than the corresponding selective catheter placement of medium. The dose is administered into a peripheral vein, the superior vena cava or right atrium, usually by mechanical injection although sometimes by rapid manual injection. The technique has been used to visualize the ventricles, aorta and most of its larger branches, including the carotids, cerebrals, vertebrals, renal, celiac, mesenterics, and the major peripheral vessels of the limbs. Radiographic visualization of these structures is possible until significant hemodilution occurs.
OMNIPAQUE 350 can be injected intravenously as a rapid bolus to provide arterial visualization using digital subtraction radiography. Preprocedural medications are not considered necessary. OMNIPAQUE 350 has provided diagnostic arterial radiographs in about 95% of patients. In some cases, poor arterial visualization has been attributed to patient movement. OMNIPAQUE 350 is very well tolerated in the vascular system. Patient discomfort (general sensation of heat and/or pain) following injection is less than with various other contrast media.
Precautions
Since the contrast medium is usually administered mechanically under high pressure, rupture of smaller peripheral veins can occur. It has been suggested that this can be avoided by using an intravenous catheter threaded proximally beyond larger tributaries or, in the case of the antecubital vein, into the superior vena cava. Sometimes the femoral vein is used. (See PRECAUTIONS—General.)
Adverse Reactions
Cardiovascular system reactions in digital arteriography included transient PVCs (16%) and PACs (6.5%). (See ADVERSE REACTIONS: Intravascular—General.)
Dosage and Administration
The usual injection volume of OMNIPAQUE 350 for the intravenous digital technique is 30 mL to 50 mL of a 350 mg Iodine/mL solution. This is administered as a bolus at 7.5 to 30 mL/second using a pressure injector. The volume and rate of injection will depend primarily on the type of equipment and technique used.
Frequently three or more injections may be required, up to a total volume not to exceed 250 mL (87.5 g Iodine).
PERIPHERAL ANGIOGRAPHY
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL or OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in adults for use in peripheral arteriography. OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in adults for use in peripheral venography.
Sedative medication may be employed prior to use. Anesthesia is not considered necessary. Patient discomfort during and immediately following injection is substantially less than that following injection of various other contrast media. Moderate to severe discomfort is very unusual.
Precautions
Pulsation should be present in the artery to be injected. In thromboangiitis obliterans, or ascending infection associated with severe ischemia, angiography should be performed with extreme caution, if at all. (See PRECAUTIONS—General.)
Adverse Reactions
A transient sensation of mild warmth is usual, immediately following injection. This has not interfered with the procedure.
In phlebography the incidence of leg pain was 21%. This usually was mild and lasted a short time after injection. (See ADVERSE REACTIONS: Intravascular—General.)
Dosage and Administration
The volume required will depend on the size, flow rate, and disease state of the injected vessel and on the size and condition of the patient, as well as the imaging technique used. The dosage recommended for use in peripheral angiography is as follows:
Aortofemoral runoffs: 20 mL to 70 mL of OMNIPAQUE 350 (350 mg Iodine/mL) 30 mL to 90 mL of OMNIPAQUE 300 (300 mg Iodine/mL) Selective arteriograms:
(femoral/iliac)10 mL to 30 mL of OMNIPAQUE 350 (350 mg Iodine/mL)
10 mL to 60 mL of OMNIPAQUE 300 (300 mg Iodine/mL)Venography (per leg): 40 mL to 100 mL of OMNIPAQUE 300 (300 mg Iodine/mL) EXCRETORY UROGRAPHY
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL or OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated for use in adults in excretory urography to provide diagnostic contrast of the urinary tract.
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in pediatric patients for excretory urography. (See Section III for information on voiding cystourethrography.)
For pharmacokinetics of excretion in adults, see CLINICAL PHARMACOLOGY—Intravascular.
Precautions
Preparatory dehydration is not recommended in the elderly, pediatric patients, diabetic or azotemic patients, or in patients with suspected myelomatosis.
Pediatric patients at higher risk of experiencing adverse events during contrast medium administration may include those having asthma, a sensitivity to medication and/or allergens, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age.
Since there is a possibility of temporary suppression of urine formation, it is recommended that a suitable interval elapse before excretory urography is repeated, especially in patients with unilateral or bilateral reduction in renal function. (See PRECAUTIONS—General.)
Dosage and Administration
Adults
OMNIPAQUE 300 and OMNIPAQUE 350 at dosages from 200 mg Iodine/kg body weight to 350 mg Iodine/kg body weight have produced diagnostic opacification of the excretory system in patients with normal renal function.
Pediatrics
Excretory Urography
OMNIPAQUE 300 at doses of 0.5 mL/kg to 3 mL/kg of body weight has produced diagnostic opacification of the excretory tract. The usual dose for pediatric patients is 1 mL/kg to 1.5 mL/kg. Dosage for pediatric patients should be administered in proportion to age and body weight. The total administered dose should not exceed 3 mL/kg.
- SECTION III
-
CLINICAL PHARMACOLOGY—OraI/Body Cavity Use
For most body cavities, the injected iohexol is absorbed into the surrounding tissue and eliminated by the kidneys and bowel as previously described in SECTION II, CLINICAL PHARMACOLOGY—Intravascular. Examinations of the uterus (hysterosalpingography) and bladder (voiding cystourethrography) involve the almost immediate drainage of contrast medium from the cavity upon conclusion of the radiographic procedure.
Orally administered iohexol is very poorly absorbed from the normal gastrointestinal tract. Only 0.1 to 0.5 percent of the oral dose was excreted by the kidneys. This amount may increase in the presence of bowel perforation or bowel obstruction. Iohexol is well tolerated and readily absorbed if leakage into the peritoneal cavity occurs.
Visualization of the joint spaces, uterus, fallopian tubes, peritoneal herniations, pancreatic and bile ducts, and bladder can be accomplished by direct injection of contrast medium into the region to be studied. The use of appropriate iodine concentrations assures diagnostic density.
Orally administered OMNIPAQUE produces good visualization of the gastrointestinal tract. OMNIPAQUE is particularly useful when barium sulfate is contraindicated as in patients with suspected bowel perforation or those where aspiration of contrast medium is a possibility.
-
INDICATIONS AND USAGE, GENERAL—Oral/Body Cavity Use
OMNIPAQUE 300 and OMNIPAQUE 350 have osmolalities from approximately 2.4 to 3 times that of plasma (285 mOsm/kg water) and are hypertonic under conditions of use.
Adults
OMNIPAQUE 350 is indicated in adults for arthrography and oral pass-thru examination of the gastrointestinal tract.
OMNIPAQUE 300 is indicated in adults for arthrography and hysterosalpingography. OMNIPAQUE diluted to concentrations from 6 mg Iodine/mL to 9 mg Iodine/mL administered orally in conjunction with OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL administered intravenously is indicated in adults for contrast enhanced computed tomography of the abdomen.
Pediatric Patients
OMNIPAQUE 300 is indicated in pediatric patients for examination of the gastrointestinal tract.
OMNIPAQUE diluted to concentrations from 50 mg Iodine/mL to 100 mg Iodine/mL is indicated in pediatric patients for voiding cystourethrography.
OMNIPAQUE diluted to concentrations from 9 mg Iodine/mL to 21 mg Iodine/mL administered orally in conjunction with OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL administered intravenously are indicated in pediatric patients for use in contrast enhanced computed tomography of the abdomen.
- CONTRAINDICATIONS
- WARNINGS—General
-
PRECAUTIONS—General
See SECTION II, PRECAUTIONS—General.
Orally administered hypertonic contrast media draw fluid into the intestines which, if severe enough, could result in hypovolemia. Likewise, in pediatric patients, the occurrence of diarrhea may result in hypovolemia. Plasma fluid loss may be sufficient to cause a shock- like state which, if untreated, could be dangerous. This is especially pertinent to the elderly, cachectic patients of any age as well as infants and small pediatric patients.
-
ADVERSE REACTIONS: Oral/Body Cavity Use—General
Body Cavities
In controlled clinical trials involving 285 adult patients for various body cavity examinations using OMNIPAQUE 300 and 350, the following adverse reactions were reported.
Cardiovascular System Incidence > 1%: None Incidence ≤ 1%: Hypertension
Nervous System
Incidence > 1%: Pain (26%)
Incidence ≤ 1%: Headache, somnolence, fever, muscle weakness, burning, unwell feeling, tremors, lightheadedness, syncope
Respiratory System
None
Gastrointestinal System
Incidence > 1%: None
Incidence ≤ 1%: Flatulence, diarrhea, nausea, vomiting, abdominal pressure
Skin and Appendages
Incidence > 1%: Swelling (22%), heat (7%)
Incidence ≤ 1%: Hematoma at injection site
The most frequent reactions, pain and swelling, were almost exclusively reported after arthrography and were generally related to the procedure rather than the contrast medium.
Gastrointestinal reactions were almost exclusively reported after oral pass-thru examinations.
For additional information on adverse reactions that may be expected with specific procedures, see INDIVIDUAL INDICATIONS AND USAGE. For information on general adverse reactions to contrast media, see SECTION II, ADVERSE REACTIONS: Intravascular—General.
No adverse reactions associated with the use of OMNIPAQUE for VCU procedures were reported in 51 pediatric patients studied.
-
OVERDOSAGE
See also SECTION II, OVERDOSAGE.
The recommended dose of OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL for adult oral pass-thru examination of the gastrointestinal tract is 50 mL to 100 mL. In a Phase I study, 150 mL of OMNIPAQUE 350 was administered orally to 11 healthy male subjects. The incidence of diarrhea was 91% (10 of 11) and abdominal cramping was 27% (3 of 11). Despite all of these events being mild and transient the occurrences were more than double that seen at the recommended doses. It is apparent from this finding that larger volumes of hypertonic contrast media, like OMNIPAQUE, increase the osmotic load in the bowel which may result in greater fluid shifts.
- DOSAGE AND ADMINISTRATION—General
-
INDIVIDUAL INDICATIONS AND USAGE
Oral Use
Adults
OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in adults for use in oral pass-thru examination of the gastrointestinal tract. OMNIPAQUE diluted to concentrations from 6 mg Iodine/mL to 9 mg Iodine/mL administered orally in conjunction with OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL administered intravenously are indicated in adults for use in contrast enhanced computed tomography of the abdomen. Dilute oral plus intravenous OMNIPAQUE may be useful when unenhanced imaging does not provide sufficient delineation between normal loops of the bowel and adjacent organs or areas of suspected pathology.
Pediatric patients
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL administered orally or rectally is indicated in pediatric patients for use in examination of the gastrointestinal tract. OMNIPAQUE diluted to concentrations from 9 mg Iodine/mL to 21 mg Iodine/mL administered orally in conjunction with OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL administered intravenously are indicated in pediatric patients for use in contrast enhanced computed tomography of the abdomen.
Adverse Reactions
Oral administration of OMNIPAQUE is most often associated with mild, transient diarrhea especially when high concentrations and large volumes are administered. Nausea, vomiting, and moderate diarrhea have also been reported following orally administered OMNIPAQUE, but much less frequently. For CT examinations using dilute oral plus intravenous contrast medium, adverse events are more likely to be associated with the intravenous injection than the hypotonic oral solution. It should be noted that serious or anaphylactoid reactions that may occur with intravascular iodinated media are possible following administration by other routes.
Adults
In controlled clinical trials involving 54 adult patients for oral pass-thru examination of the gastrointestinal tract using OMNIPAQUE 350, the following adverse reactions were reported:
diarrhea (42%), nausea (15%), vomiting (11%), abdominal pain (7%), flatulence (2%), and headache (2%). In controlled clinical studies involving 44 adult patients for dilute oral plus intravenous CT examination of the gastrointestinal tract using OMNIPAQUE 300, adverse reactions were limited to a single report of vomiting (2%).
Pediatric patients
In controlled clinical studies involving 58 pediatric patients for examination of the gastrointestinal tract at a concentration of 300 mg Iodine/mL, the following adverse reactions were reported: diarrhea (36%), vomiting (9%), nausea (5%), fever (5%), hypotension (2%), abdominal pain (2%), and urticaria (2%). In clinical studies an increased frequency and severity of diarrhea was noted with an increase in the administered concentration and dose of the radiocontrast agent. In controlled clinical studies involving 69 pediatric patients for dilute oral plus intravenous CT examination of the gastrointestinal tract using OMNIPAQUE 300, adverse reactions were limited to a single report of vomiting (1.4%).
Dosage and Administration
Adults
The recommended dosage of undiluted OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL for oral pass-thru examination of the gastrointestinal tract in adults is 50 mL to 100 mL depending on the nature of the examination and the size of the patient. The recommended oral dosage of OMNIPAQUE diluted to concentrations of 6 mg Iodine/mL to 9 mg Iodine/mL for contrast enhanced computed tomography of the abdomen in adults is 500 mL to 1000 mL. Smaller administered volumes are needed as the concentration of the final solution is increased (see Table below). In conjunction with dilute oral administration, the recommended dosage of OMNIPAQUE 300 intravenously is 100 mL to 150 mL. The oral dose is administered about 20 to 40 minutes prior to the intravenous dose and image acquisition.
Pediatric Patients
The dosage of undiluted OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL, is dependent on the nature of the examination and the size of the patient. Based on clinical experience, it is recommended that OMNIPAQUE 180 (available in single use vials), be used in pediatric patients less than 3 months of age. OMNIPAQUE 300 may be used in pediatric patients 3 months of age and older.
The following dosage guidelines are recommended:
Age Volume of OMNIPAQUE Less than 3 months 5 — 30 mL Three months to 3 years Up to 60 mL Four years to 10 years Up to 80 mL Greater than 10 years Up to 100 mL When given rectally, larger volumes may be used.
The recommended oral dosage of OMNIPAQUE diluted to concentrations of 9 mg Iodine/mL to 21 mg Iodine/mL for contrast computed tomography of the abdomen in pediatric patients is 180 mL to 750 mL. Smaller administered volumes are needed as the concentration of the final solution is increased (see Table below). The total oral dose in grams of iodine should generally not exceed 5 g Iodine for pediatric patients under 3 years of age and 10 g Iodine for pediatric patients from 3 to 18 years of age. The oral dosage may be given all at once or over a period of 30 to 45 minutes if there is difficulty in consuming the required volume. In conjunction with dilute oral administration the recommended dosage of OMNIPAQUE 300 is 2mL/kg when administered intravenously with a range of 1 mL/kg to 2 mL/kg. Dosage pediatric patients should be administered in proportion to age and body weight. The total intravenously administered dose should not exceed 3 mL/kg. The oral dose is administered about 30 to 60 minutes prior to the intravenous dose and image acquisition.
OMNIPAQUE may be diluted with water or beverage as follows:
To Achieve Add To One Liter of Contrast Medium at A Final Concentration
(mg Iodine/mL) ofStock Concentration of OMNIPAQUE
(mg Iodine/mL)Volume
(mL)Water, Carbonated Beverage, Milk, or Juice
(mL)6 300 20 980 350 17 983 9 300 30 970 350 26 974 12 300 40 960 350 35 965 15 300 50 950 350 43 957 18 300 60 940 350 52 948 21 300 70 930 350 60 940 Dilutions of OMNIPAQUE should be prepared just prior to use and any unused portion discarded after the procedure.
VOIDING CYSTOURETHROGRAPHY (VCU)
OMNIPAQUE diluted to concentrations from 50 mg Iodine/mL to 100 mg Iodine/mL is indicated in pediatric patients for voiding cystourethrography. VCUs are often performed in conjunction with excretory urography.
Precautions
See PRECAUTIONS—General.
Since the VCU procedure requires instrumentation, special precautions should be observed in those patients known to have an acute urinary tract infection. Filling of the bladder should be done at a steady rate, exercising caution to avoid excessive pressure. Sterile procedures are essential.
Dosage and Administration
OMNIPAQUE may be diluted, utilizing aseptic technique, with Sterile Water for Injection to a concentration of 50 mg Iodine/mL to 100 mg Iodine/mL for voiding cystourethrography. The concentration may vary depending upon the patient's size and age and also with the technique and equipment used. Sufficient volume of contrast medium should be administered to adequately fill the bladder. The usual volume ranges from 50 mL to 300 mL of OMNIPAQUE at a concentration of 100 mg Iodine/mL and 50 mL to 600 mL of OMNIPAQUE at a concentration of 50 mg Iodine/mL.
OMNIPAQUE may be diluted with Sterile Water for Injection as indicated in the table below:
To Achieve Add To A Final Concentration Each 100 mL of OMNIPAQUE Sterile Water for Injection, USP (mL) (mg Iodine/mL) OMNIPAQUE 300 OMNIPAQUE 350 100 200 250 90 233 289 80 275 338 70 330 400 60 400 483 50 500 600 Dilutions of OMNIPAQUE should be prepared just prior to use and any unused portion discarded after the procedure.
ARTHROGRAPHY
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL or OMNIPAQUE 350 at a concentration of 350 mg Iodine/mL is indicated in radiography of the knee joint in adults, and OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in radiography of the shoulder joint in adults, and OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in radiography of the temporomandibular joint in adults. Arthrography may be helpful in the diagnosis of posttraumatic or degenerative joint diseases, synovial rupture, the visualization of communicating bursae or cysts, and in meniscography.
Precautions
See PRECAUTIONS—General.
Strict aseptic technique is required to prevent infection. Fluoroscopic control should be used to ensure proper needle placement, prevent extracapsular injection, and prevent dilution of contrast medium. Undue pressure should not be exerted during injection.
Adverse Reactions
Injection of OMNIPAQUE into the joint is associated with transient discomfort, ie, pain, swelling. However, delayed, severe or persistent discomfort may occur occasionally. Severe pain may often result from undue use of pressure or the injection of large volumes. Joint swelling after injection is less with OMNIPAQUE than with high osmolar ionic contrast medium. These types of reactions are generally procedurally dependent and of greater frequency when double-contrast technique is employed.
Nervous system: Swelling sensation (42%), pain (29%), heat sensation (13%), and muscle weakness (0.7%).
Skin and appendages: Hematoma at injection site (0.7%).
Dosage and Administration
Arthrography is usually performed under local anesthesia. The amount of OMNIPAQUE injected is dependent on the size of the joint to be examined and the technique employed. Lower volumes of contrast medium are usually injected for knee and shoulder arthrography when double-contrast examinations using 15 mL to 100 mL of air are performed.
The following concentrations and volumes are recommended for normal adult knee, shoulder, and temporomandibular joints but should serve as guidelines since joints may require more or less contrast medium for optimal visualization.
KNEE
OMNIPAQUE 300 5 mL to 15 mL
OMNIPAQUE 350 5 mL to 10 mL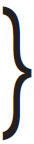
Lower volumes recommended for double-contrast examinations; higher volumes recommended for single-contrast examinations. SHOULDER
OMNIPAQUE 300 10 mLTEMPOROMANDIBULAR
OMNIPAQUE 300 0.5 mL to 1 mLPassive or active manipulation is used to disperse the medium throughout the joint space.
HYSTEROSALPINGOGRAPHY
OMNIPAQUE 300 at a concentration of 300 mg Iodine/mL is indicated in radiography of the internal group of adult female reproductive organs; ovaries, fallopian tubes, uterus, and vagina. Hysterosalpingography is utilized as a diagnostic and therapeutic modality in the treatment of infertility and other abnormal gynecological conditions.
Contraindications
OMNIPAQUE 300 for hysterosalpingography is contraindicated during pregnancy or suspected pregnancy, menstruation or when menstruation is imminent , within 6 months after termination of pregnancy, within 30 days after conization or curettage, when signs of infection are present in any portion of the genital tract including the external genitalia, and when reproductive tract neoplasia is known or suspected because of the risk of peritoneal spread of neoplasm.
Adverse Reactions
Injection of OMNIPAQUE for hysterosalpingography is associated with immediate, transient pain. Monitor injection pressure and volume instilled to minimize pain and to avoid disruptive distention of the uterus and fallopian tubes. Fluoroscopic monitoring is recommended.
Nervous system: Pain (49%), somnolence and fever each with an individual incidence of 3%.
Gastrointestinal system: Nausea (3%).
-
DIRECTIONS FOR USE
- The transfer of OMNIPAQUE (Iohexol Injection) from the Pharmacy Bulk Package is restricted to a suitable work area, such as a laminar flow hood.
- The container closure may be penetrated only one time, utilizing a suitable transfer device and aseptic technique.
- The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of 8 hours from initial closure entry is permitted to complete fluid transfer operations. The container should not be removed from the aseptic area during the entire 8 hour period.
- The temperature of the container should not exceed 37°C, after the closure has been entered.
-
HOW SUPPLIED
OMNIPAQUE 300
500 mL in + PLUSPAK™ (polymer bottle), boxes of 10 Pharmacy Bulk Packages
(NDC 0407-1413-89)OMNIPAQUE 350
500 mL in + PLUSPAK™ (polymer bottle), boxes of 10 Pharmacy Bulk Packages
(NDC 0407-1414-88)Protect polymer bottles of OMNIPAQUE from strong daylight and direct exposure to sunlight. Do not freeze. OMNIPAQUE should be stored at controlled room temperature, 20°-25°C (68°- 77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
OMNIPAQUE Injection in all presentations may be stored in a contrast media warmer for up to one month at 37°C (98.6°F).
SPECIAL HANDLING AND STORAGE FOR POLYMER BOTTLES ONLY: DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING.
-
SPL UNCLASSIFIED SECTION
NOVAPLUS ®
Distributed by
GE Healthcare Inc.,
Marlborough, MA 01752 U.S.A.Product of Norwegian Origin.
Omnipaque is a trademark of GE HealthCare or one of its subsidiaries.
GE is a trademark of General Electric Company used under trademark license.
NOVAPLUS is a registered trademark of Vizient, Inc.© 2023 GE HealthCare
Revised 4/2023
- PRINCIPAL DISPLAY PANEL - 300 mg Bottle Label
- PRINCIPAL DISPLAY PANEL - 350 mg Bottle Label
-
INGREDIENTS AND APPEARANCE
OMNIPAQUE
iohexol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0407-1413 Route of Administration INTRAVENOUS, INTRAVASCULAR, INTRATHECAL, ORAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOHEXOL (UNII: 4419T9MX03) (IOHEXOL - UNII:4419T9MX03) IODINE 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) HYDROCHLORIC ACID (UNII: QTT17582CB) Product Characteristics Color yellow (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0407-1413-89 10 in 1 BOX 08/03/2005 10/22/2024 1 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020608 08/03/2005 OMNIPAQUE
iohexol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0407-1414 Route of Administration INTRAVENOUS, INTRAVASCULAR, ORAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOHEXOL (UNII: 4419T9MX03) (IOHEXOL - UNII:4419T9MX03) IODINE 350 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) HYDROCHLORIC ACID (UNII: QTT17582CB) Product Characteristics Color yellow (colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0407-1414-88 10 in 1 BOX 08/03/2005 11/08/2024 1 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020608 08/03/2005 Labeler - GE Healthcare Inc. (053046579) Establishment Name Address ID/FEI Business Operations GE Healthcare Lindesnes 518890970 api manufacture(0407-1413, 0407-1414) Establishment Name Address ID/FEI Business Operations GE Healthcare Shanghai Co., Ltd. 545292716 manufacture(0407-1413, 0407-1414) Establishment Name Address ID/FEI Business Operations GE Healthcare Ireland Limited 988006565 manufacture(0407-1413, 0407-1414)