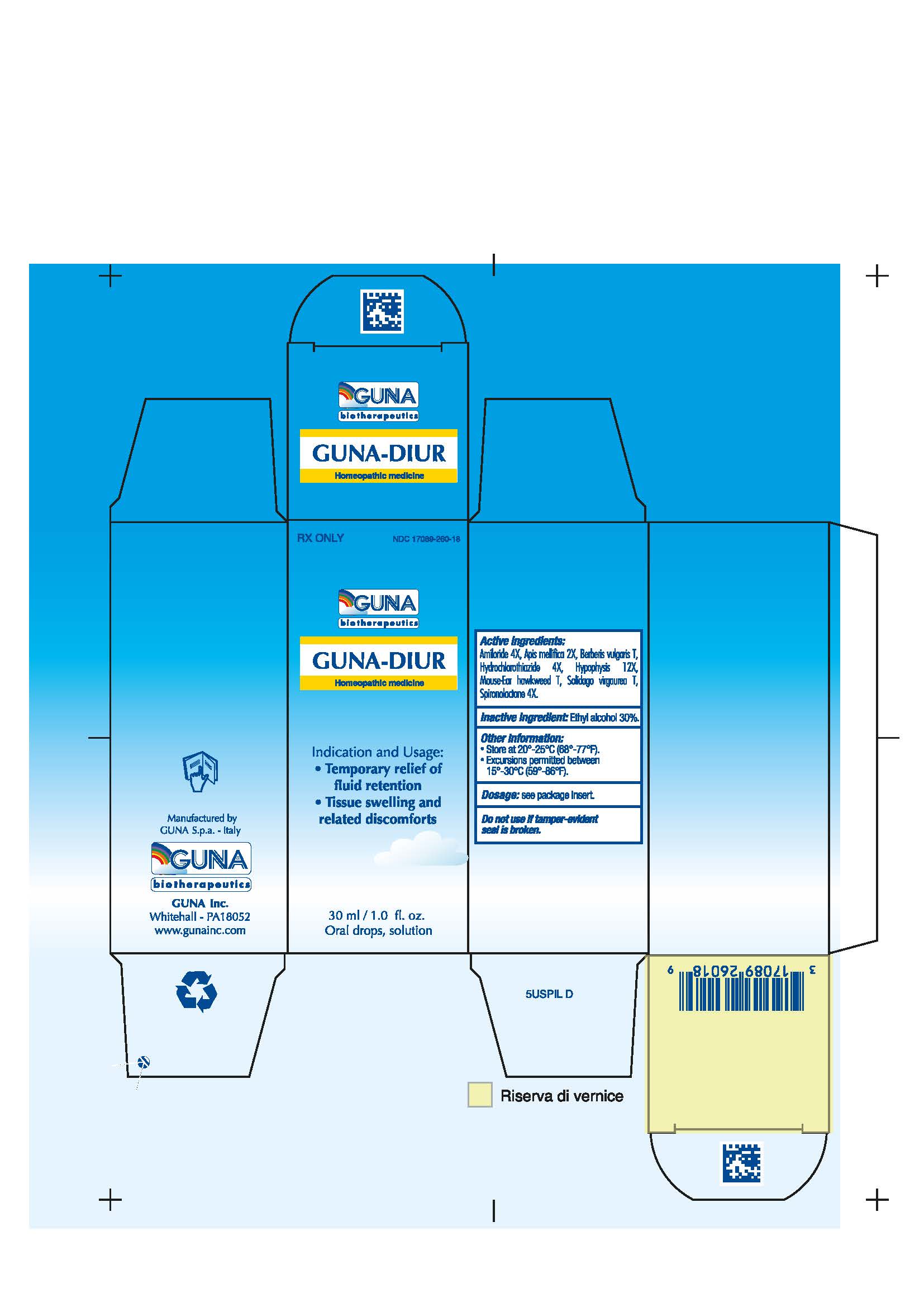
Label: GUNA-DIUR- amiloride - apis mellifera - berberis vulgaris fruit - hieracium pilosella flowering top - hydrochlorothiazide - solidago virgaurea flowering top - spironolactone - sus scrofa pituitary gland - solution/ drops
- NDC Code(s): 17089-260-18
- Packager: Guna spa
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 21, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- 1. INDICATIONS AND USAGE
- 2. DOSAGE AND ADMINISTRATION
-
3. DOSAGE FORMS AND STRENGTHS
3.1. 30 ml Bottle dropper container contains:
Active ingredients: Amiloride 4X 0.006 ml, Apis Mellifica 2X 0.626 ml, Berberis Vulgaris T 0.314 ml, Hydrochlorothiazide 4X 0.006 ml, Hypophysis 12X 6.314 ml, Mouse-Ear Hawkweed T 6.314 ml, Solidago Virgaurea T 0.314 ml, Spironolactone 4X 0.006 ml.
Inactive Ingredient: Ethylic Alcohol 30%
- 4. CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA-DIUR. GUNA®- DIUR should not be given to a pregnant woman.
8.2. Lactation: It is not known whether any of the ingredients in GUNA- DIUR are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA- DIUR is administered to a nursing woman.
8.3. Pediatric use: Safety and effectiveness in pediatric patients have not been established.
8.4. Geriatric use: No restrictions.
- 9. DRUG ABUSE AND DEPENDENCE
- 10. OVERDOSAGE
- 11. DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 15. REFERENCES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
- 17. PATIENT COUNSELING INFORMATION
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
GUNA-DIUR
amiloride - apis mellifera - berberis vulgaris fruit - hieracium pilosella flowering top - hydrochlorothiazide - solidago virgaurea flowering top - spironolactone - sus scrofa pituitary gland - solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17089-260 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMILORIDE (UNII: 7DZO8EB0Z3) (AMILORIDE - UNII:7DZO8EB0Z3) AMILORIDE 4 [hp_X] in 30 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 2 [hp_X] in 30 mL BERBERIS VULGARIS FRUIT (UNII: 6XEF22AHC3) (BERBERIS VULGARIS FRUIT - UNII:6XEF22AHC3) BERBERIS VULGARIS FRUIT 0.3 g in 30 mL HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 4 [hp_X] in 30 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 12 [hp_X] in 30 mL HIERACIUM PILOSELLA FLOWERING TOP (UNII: 08A7Y81S1P) (HIERACIUM PILOSELLA FLOWERING TOP - UNII:08A7Y81S1P) HIERACIUM PILOSELLA FLOWERING TOP 0.3 g in 30 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 0.3 g in 30 mL SPIRONOLACTONE (UNII: 27O7W4T232) (SPIRONOLACTONE - UNII:27O7W4T232) SPIRONOLACTONE 4 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-260-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-260)