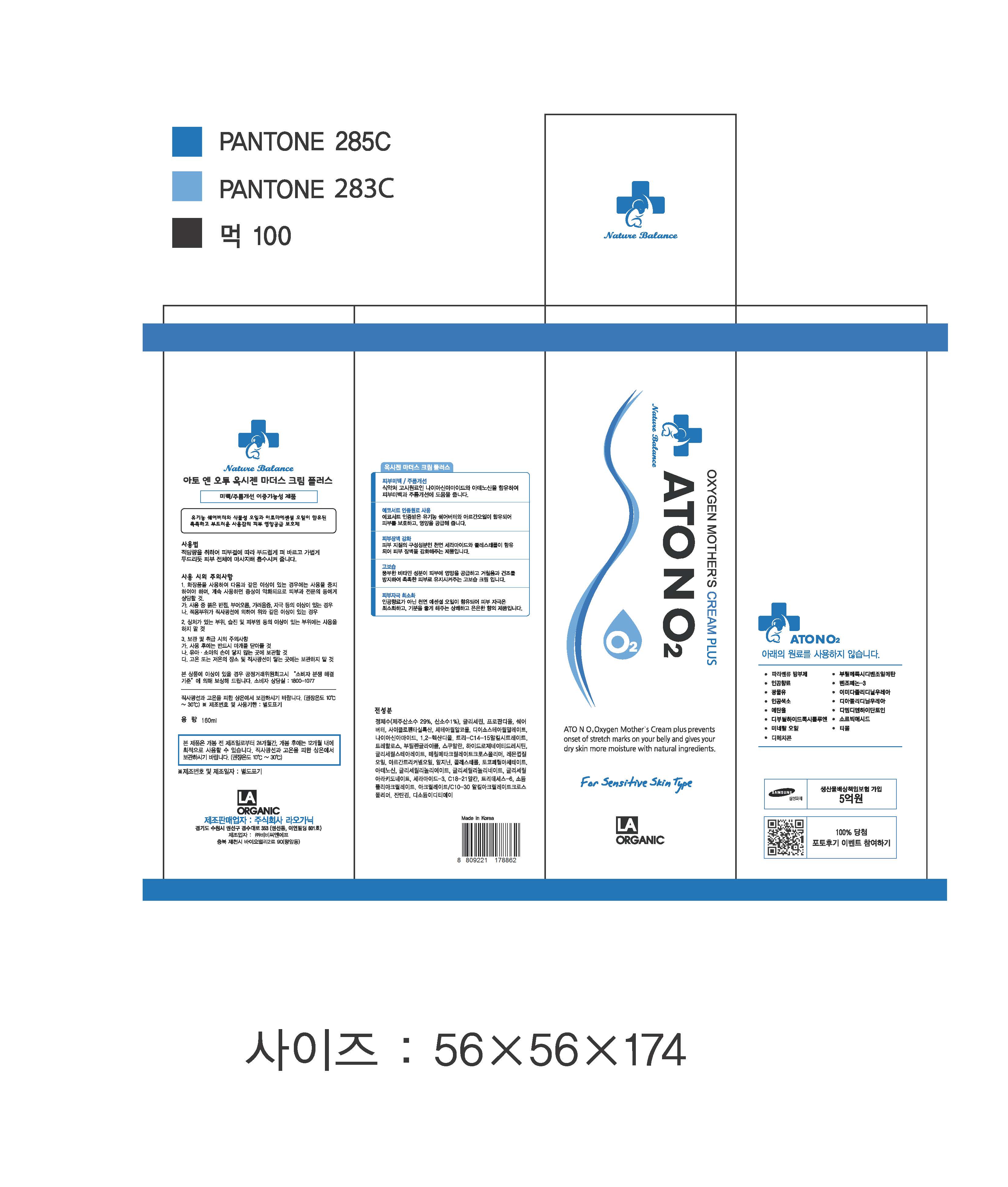
Label: ATONO2 OXYGEN MOTHERS CREAM- niacinamide , adenosine cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70993-0001-1 - Packager: LAORGANIC Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 11, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
- For external use only
- When using this product
1. If any one of following symptoms occurs when using cosmetics, stop using it and consult a dermatologist.
A. red spot, swelling, itching, stimulus
B. symptoms mentioned above are caused by sunlight.2. Do not apply on skin where there is wound, eczema, or irritation.
3. Storage and cautions for handling
A. Close stopper after using.
B. Keep out of reach of children.
C. Keep away from high or low temperature and direct sunlight.4. This is not a medicinal product. Made of 100% cosmetic ingredients, it is a sensitive-skin care product for all skin types.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ATONO2 OXYGEN MOTHERS CREAM
niacinamide , adenosine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70993-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2 g in 100 mL ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70993-0001-1 160 mL in 1 TUBE; Type 0: Not a Combination Product 09/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/01/2014 Labeler - LAORGANIC Co., Ltd. (689844148) Registrant - LAORGANIC Co., Ltd. (689844148) Establishment Name Address ID/FEI Business Operations LAORGANIC Co., Ltd. 689844148 manufacture(70993-0001)