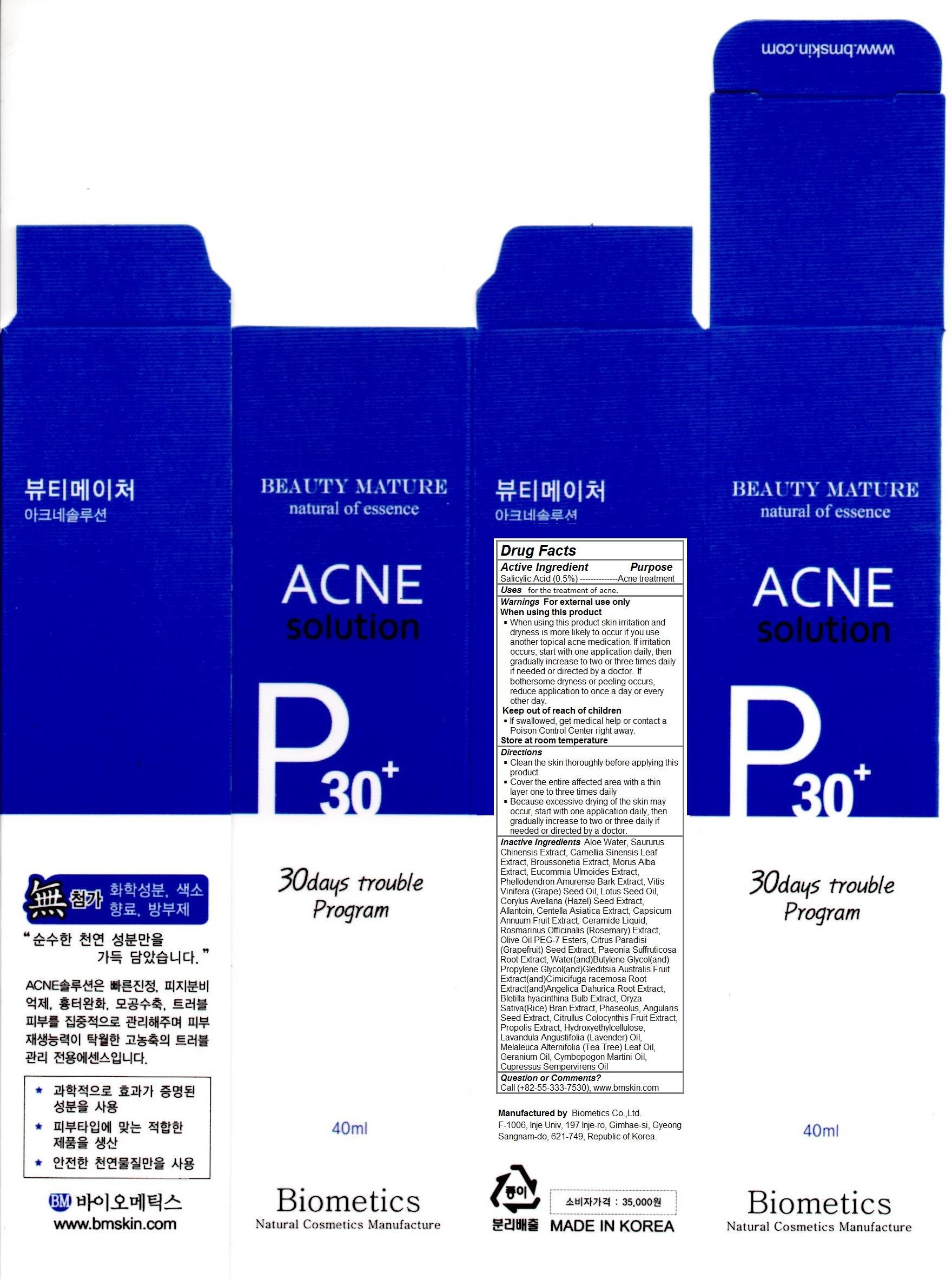
Label: ACNE SOLUTION- salicylic acid solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 62818-101-01, 62818-101-02 - Packager: Biometics Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 16, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Acne Solution
- Pyrithione Zinc
- for the treatment of acne
- Keep out of reach of children If swallowed, get medical help or contact a Poison Control Center right away.
- Directions Clean the skin thoroughly before applying this product. Cover the entire affected area with a thin layer one to three times daily Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three daily if needed or directed by a doctor.
- Warnings For external use only When using this product When using this product skin irritation and dryness is more likely to occur if you use another topical acne medication. If irritation occurs, start with one application daily, then gradually increase to two or three times daily if needed or directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three daily if needed or directed by a doctor.
- Aloe Water, Saururus Chinensis Extract, Camellia Sinensis Leaf Extract, Broussonetia Extract, Morus Alba Extract, Eucommia Ulmoides Extract, Phellodendron Amurense Bark Extract, Vitis Vinifera (Grape) Seed Oil, Lotus Seed Oil, Corylus Avellana (Hazel) Seed Extract, Allantoin, Centella Asiatica Extract, Capsicum Annuum Fruit Extract, Ceramide Liquid, Rosmarinus Officinalis (Rosemary) Extract, Olive Oil PEG-7 Esters, Citrus Paradisi (Grapefruit) Seed Extract, Paeonia Suffruticosa Root Extract, Water(and)Butylene Glycol(and)Propylene Glycol(and)Gleditsia Australis Fruit Extract(and)Cimicifuga racemosa Root Extract(and)Angelica Dahurica Root Extract, Bletilla hyacinthina Bulb Extract, Oryza Sativa(Rice) Bran Extract, Phaseolus, Angularis Seed Extract, Citrullus Colocynthis Fruit Extract, Propolis Extract, Hydroxyethylcellulose, Lavandula Angustifolia (Lavender) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Geranium Oil, Cymbopogon Martini Oil, Cupressus Sempervirens Oil
-
INGREDIENTS AND APPEARANCE
ACNE SOLUTION
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62818-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) salicylic acid 0.2 g in 40 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) SAURURUS CHINENSIS WHOLE (UNII: 6DRV3D37XS) CAMELLIA SINENSIS WHOLE (UNII: C5M4585ZBZ) BROUSSONETIA PAPYRIFERA WHOLE (UNII: 0QW22BU678) MORUS ALBA WHOLE (UNII: C4228R0MX2) EUCOMMIA ULMOIDES WHOLE (UNII: 21176878UY) PHELLODENDRON AMURENSE BARK (UNII: PBG27B754G) VITIS VINIFERA SEED (UNII: C34U15ICXA) LOTUS CORNICULATUS FLOWER VOLATILE OIL (UNII: W11376RO0T) CORYLUS AVELLANA POLLEN EXTRACT (UNII: CR2J49TL6L) ALLANTOIN (UNII: 344S277G0Z) CENTELLA ASIATICA (UNII: 7M867G6T1U) CAPSICUM ANNUUM WHOLE (UNII: 7FKZ3QQQ1F) ROSMARINUS OFFICINALIS WHOLE (UNII: EA3289138M) OLIVE OIL (UNII: 6UYK2W1W1E) CITRUS PARADISI SEED (UNII: 12F08874Y7) PAEONIA SUFFRUTICOSA ROOT (UNII: 7M7E9A2C8J) PROPOLIS WAX (UNII: 6Y8XYV2NOF) CETYL HYDROXYETHYLCELLULOSE (350000 MW) (UNII: T7SWE4S2TT) LAVANDULA ANGUSTIFOLIA WHOLE (UNII: 51217XIL5L) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) CYMBOPOGON MARTINI WHOLE (UNII: 267NPH931L) CUPRESSUS SEMPERVIRENS LEAF OIL (UNII: M7QUY89S4O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62818-101-02 1 in 1 BOX 1 NDC:62818-101-01 40 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 05/01/2014 Labeler - Biometics Co.,Ltd. (689048850) Registrant - Biometics Co.,Ltd. (689048850) Establishment Name Address ID/FEI Business Operations Biometics Co.,Ltd. 689048850 manufacture(62818-101)