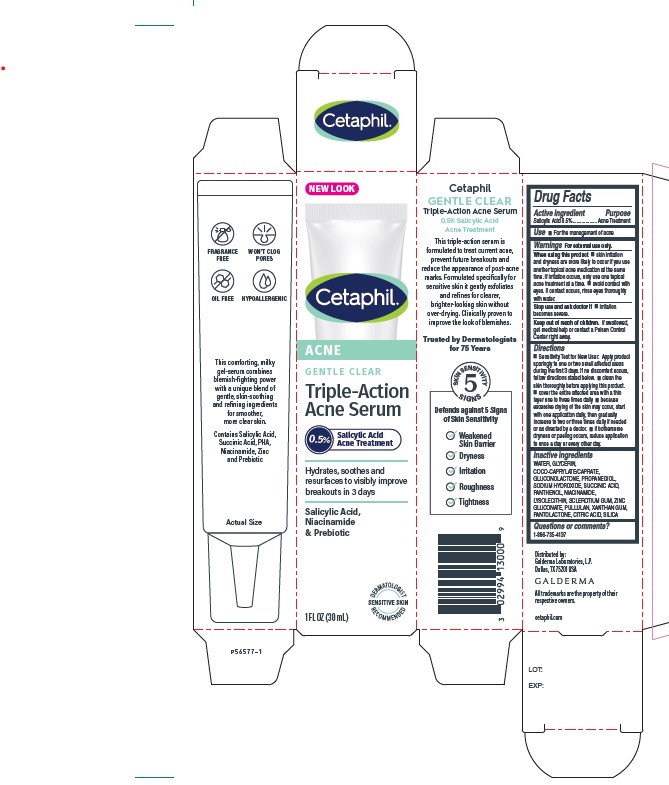
Label: CETAPHIL GENTLE CLEAR TRIPLE-ACTION ACNE SERUM- salicylic acid solution
- NDC Code(s): 0299-4135-00, 0299-4135-05
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
■ skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne treatment at a time. ■ avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.Stop use and ask a doctor if
■ irritation becomes severe - KEEP OUT OF REACH OF CHILDREN
-
Directions
•Sensitivity Test for New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow directions stated below. • clean the skin thoroughly before applying this product. • cover the entire affected area with a thin layer one to three times daily • because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. • if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Inactive Ingredients
- STORAGE AND HANDLING
- Questions or comments?
- QUESTIONS
- Principle Display Panel - 1 FL OZ Carton
-
INGREDIENTS AND APPEARANCE
CETAPHIL GENTLE CLEAR TRIPLE-ACTION ACNE SERUM
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Coco-Caprylate/Caprate (UNII: 8D9H4QU99H) Gluconolactone (UNII: WQ29KQ9POT) Propanediol (UNII: 5965N8W85T) Sodium Hydroxide (UNII: 55X04QC32I) Succinic Acid (UNII: AB6MNQ6J6L) Panthenol (UNII: WV9CM0O67Z) Niacinamide (UNII: 25X51I8RD4) Lysophosphatidylcholine, Soybean (UNII: CQD833204Z) Betasizofiran (UNII: 2X51AD1X3T) Zinc Gluconate (UNII: U6WSN5SQ1Z) Pullulan (UNII: 8ZQ0AYU1TT) Xanthan Gum (UNII: TTV12P4NEE) Pantolactone, (R)- (UNII: J288D7O0JS) Citric Acid Monohydrate (UNII: 2968PHW8QP) Silicon Dioxide (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4135-00 1 in 1 CARTON 05/01/2022 1 30 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0299-4135-05 5 mL in 1 TUBE; Type 0: Not a Combination Product 05/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 05/01/2022 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Fruit of The Earth, Inc. 080086802 manufacture(0299-4135)