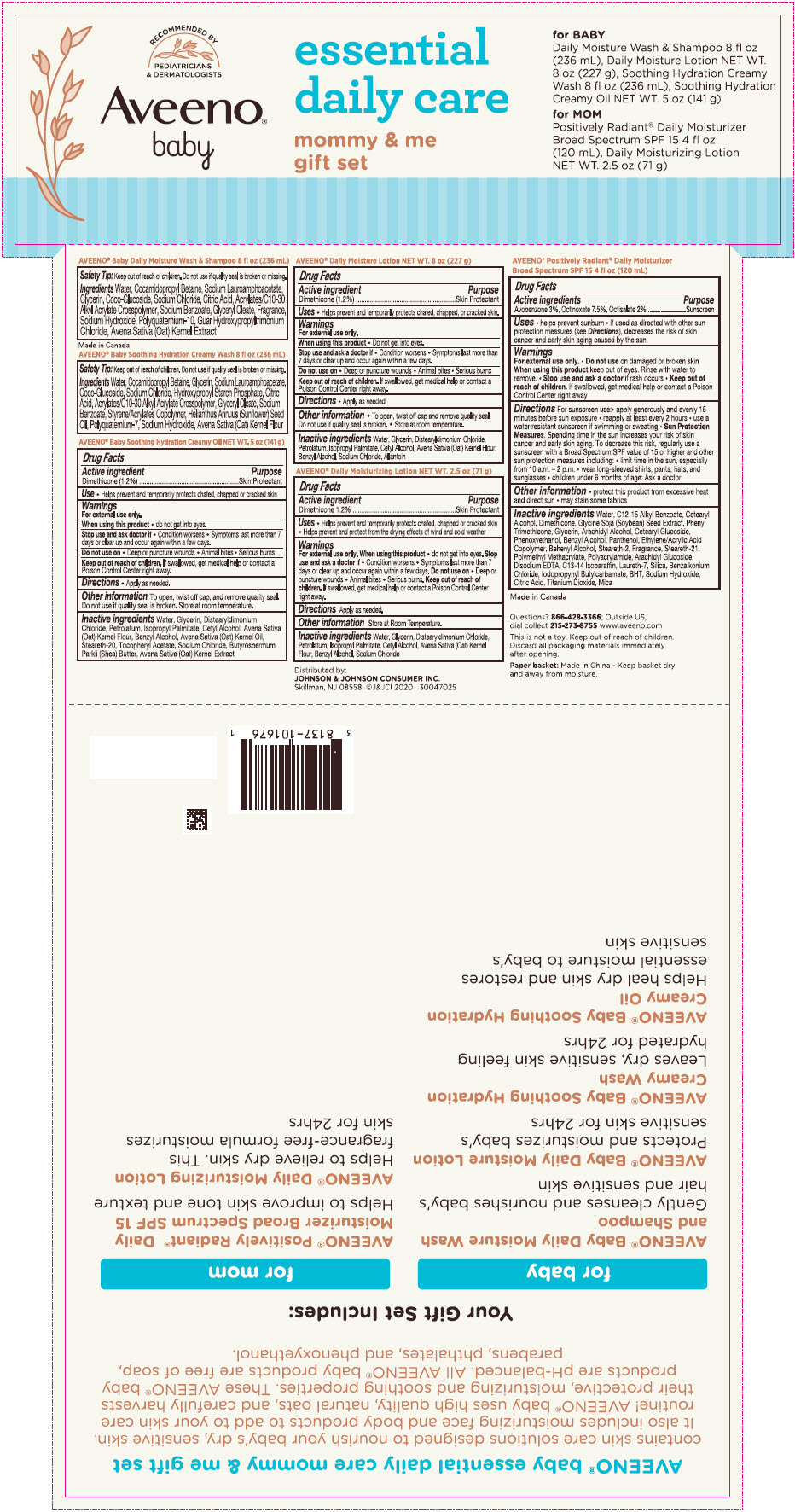
Aveeno
® baby soothing hydration creamy oil
Drug Facts
Active ingredient
Dimethicone 1.2%
Uses
- Helps prevent and temporarily protects chafed, chapped or cracked skin
Warnings
For external use only
Stop use and ask a doctor if
- Condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days
Do not use on
- Deep or puncture wounds
- Animal bites
- Serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- To open, twist off cap and remove quality seal. Do not use if quality seal is broken.
- Store at room temperature.
Inactive ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Avena Sativa (Oat) Kernel Oil, Steareth-20, Tocopheryl Acetate, Sodium Chloride, Butyrospermum Parkii (Shea) Butter, Avena Sativa (Oat) Kernel Extract.
Questions?
866-428-3366; Outside US, dial collect
215-273-8755 www.aveeno.com
Aveeno
® baby daily moisture lotion
Drug Facts
Active ingredient
Dimethicone 1.2%
Uses
- Helps prevent and temporarily protects chafed, chapped or cracked skin
Warnings
For external use only
Stop use and ask a doctor if
- Condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days
Do not use on
- Deep or puncture wounds
- Animal bites
- Serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- To open, twist off cap and remove quality seal. Do not use if quality seal is broken.
- Store at room temperature.
Inactive ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Sodium Chloride, Allantoin
Questions?
866-428-3366; Outside US, dial collect
215-273-8755 www.aveeno.com
Aveeno DAILY MOISTURIZING lotion
Drug Facts
Active ingredient
Dimethicone (1.2%)
Uses
- Helps prevent and temporarily protects chafed, chapped or cracked skin
- Helps prevent and protect from the drying effects of wind and cold weather
Warnings
Stop use and ask a doctor if
- Condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days.
Do not use on
- Deep or puncture wounds
- Animal bites
- Serious burns.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply as needed.
Other information
Store at Room Temperature
Inactive ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Sodium Chloride
Questions?
866-428-3366; Outside US, dial collect
215-273-8755 www.aveeno.com
Aveeno
® POSITIVELY RADIANT
® daily moisturizer sunscreen BROAD SPECTRUM SPF 15
Drug Facts
| Active ingredient | Purpose |
| Avobenzone 3% | Sunscreen |
| Octinoxate 7.5% | Sunscreen |
| Octisalate 2 % | Sunscreen |
Use
- helps prevent sunburn
- if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer an early skin aging caused by the sun
Warnings
-
Do not use on damaged or broken skin
-
When using this product keep out of eyes. Rinse with water to remove
-
Stop use and ask a doctor if rash occurs
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
Other information
- protect this product from excessive heat and direct sun
- may stain some fabrics
Inactive ingredients
Water, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Dimethicone, Glycine Soja (Soyabean) Seed Extract, Phenyl Trimethicone, Glycerin, Arachidyl Alcohol, Cetearyl Glycoside, Phenoxyethanol, Benzyl Alcohol, Panthenol, Ethylene/Acrylic Acid Copolymer, Behenyl Alcohol, Steareth-2, Fragrance, Steareth-21, Polymethyl Methacrylate, Polyacrylamide, Arachidyl Glucoside, Disodium EDTA, C13-14 Isoparaffin, Laureth-7, Silica, Benzalkonium Chloride, Iodopropynyl Butylcarbamate, BHT, Sodium Hydroxide, Citric Acid, Titanium Dioxide, Mica
Questions?
866-428-3366; Outside US, dial collect
215-273-8755 www.aveeno.com
Distributed by:
JOHNSON & JOHNSON CONSUMER INC.
Skillman, NJ 08558
PRINCIPAL DISPLAY PANEL - Kit Package
RECOMMENDED BY
PEDIATRICIANS
& DERMATOLOGISTS
Aveeno
®
baby
essential
daily care
mommy & me
gift set
for BABY
Daily Moisture Wash & Shampoo 8 fl oz
(236 mL), Daily Moisture Lotion NET WT.
8 oz (227 g), Soothing Hydration Creamy
Wash 8 fl oz (236 mL), Soothing Hydration
Creamy Oil NET WT. 5 oz (141 g)
FOR MOM
Positively Radiant
® Daily Moisturizer
Broad Spectrum SPF 15 4 fl oz
(120 mL), Daily Moisturizing Lotion
NET WT. 2.5 oz (71 g)
Johnson & Johnson Consumer Inc.