Label: LORATADINE tablet, orally disintegrating
-
Contains inactivated NDC Code(s)
NDC Code(s): 46122-140-52 - Packager: Good Neighbor Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 6, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
-
WARNINGS
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
- DIRECTIONS
-
OTHER INFORMATION
- Phenylketonurics: Contains Phenylalanine 0.6 mg Per Tablet.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25° C (68° to 77° F). Protect from excessive moisture.
- keep in a dry place.
- use tablet immediately after opening individual blister.
- INACTIVE INGREDIENTS
- QUESTIONS?
-
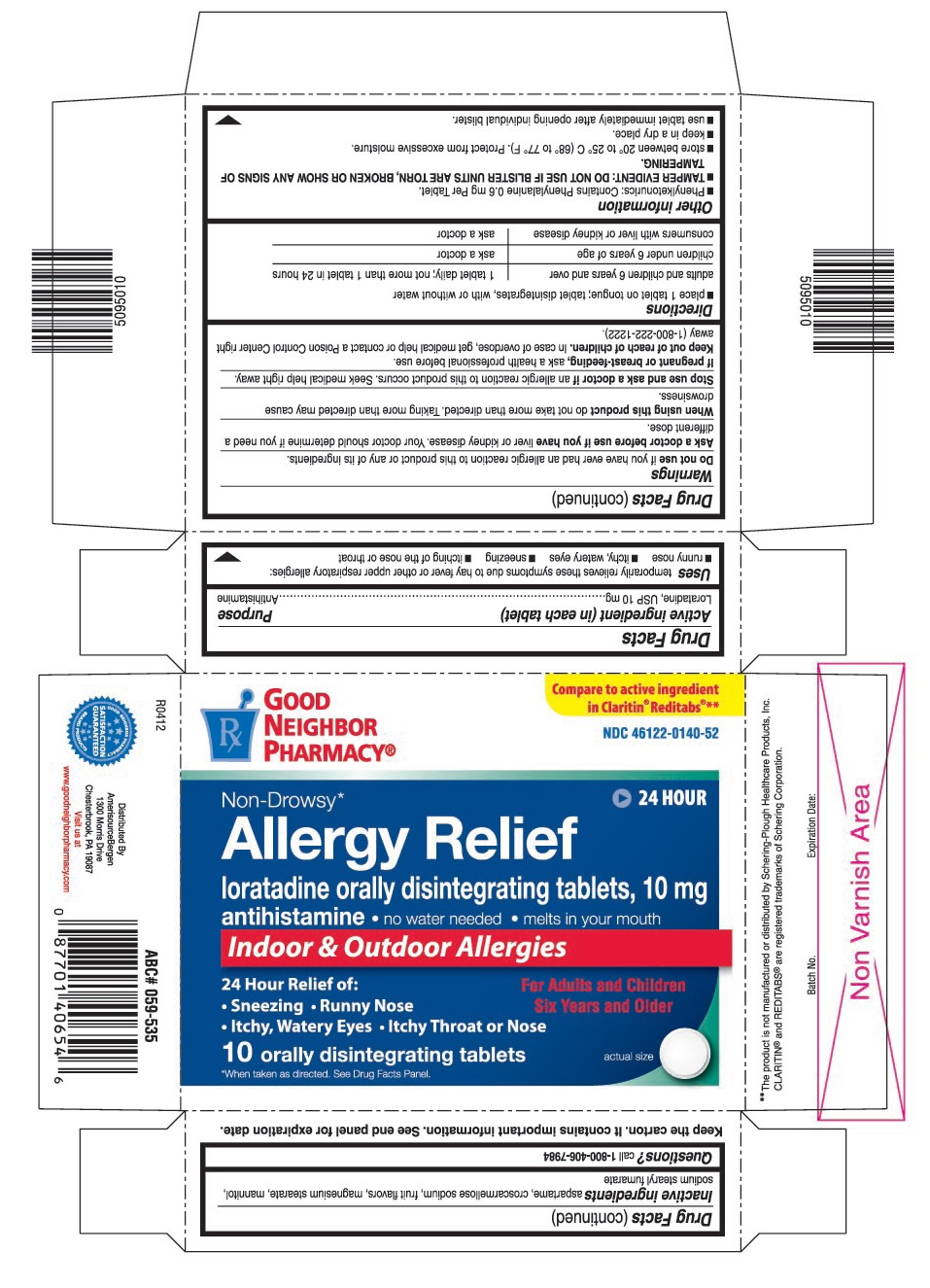
PRINCIPAL DISPLAY PANEL
Compare to active ingredient in Claritin®Reditabs®**
loratadine orally disintegrating tablets, 10 mg
- no water needed
- melts in your mouth
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
For Adults and Children Six Years and Older
10 orally disintegrating tablets
*When taken as directed. See Drug Facts Panel.
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color white (White to Off-White) Score no score Shape ROUND (Flat Faced Beveled Edge) Size 10mm Flavor FRUIT Imprint Code RC17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-140-52 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077153 08/31/2007 Labeler - Good Neighbor Pharmacy (007914906) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 manufacture(46122-140)