PRANICURA- glycerin, kaolin, calamine, menthol cream
PRANICURA GROUP LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
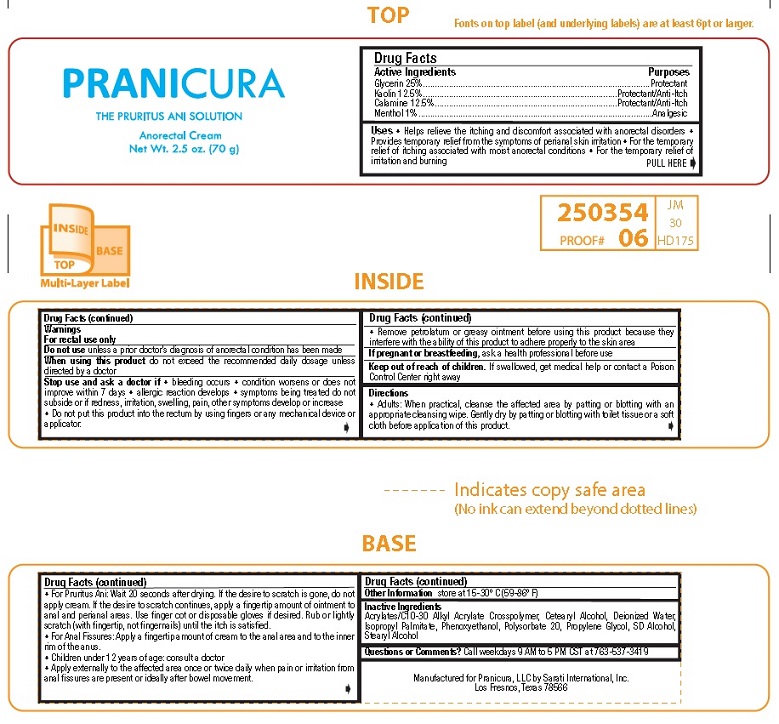
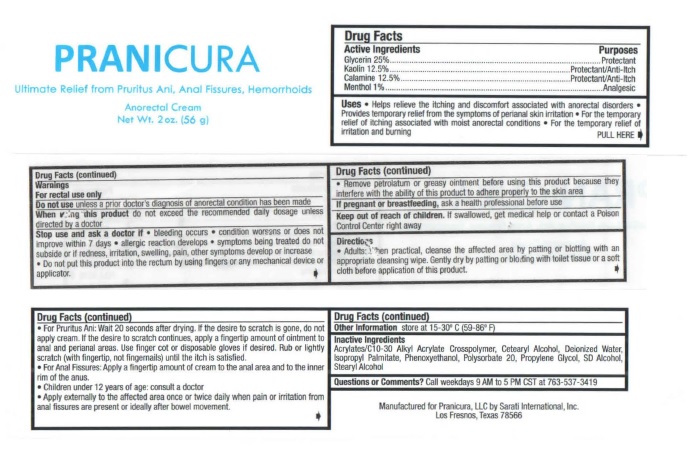
Purposes
Glycerin ..........Protectant
Kaolin .............Protectant/Anti-Itch
Calamine .........Protectant/Anti-Itch
Menthol ...........Analgesic
Uses • Helps relieve the itching and discomfort associated with anorectal disorders •
Provides temporary relief from the symptoms of perianal skin irritation • For the temporary
relief of itching associated with moist anorectal conditions • For the temporary relief of
irritation and burning
Stop use and ask a doctor if • bleeding occurs • condition worsens or does not
improve within 7 days • allergic reaction develops • symptoms being treated do not
subside or if redness, irritation, swelling, pain, other symptoms develop or increase
• Do not put this product into the rectum by using fingers or any mechanical device or
applicator.
• Remove petrolatum or greasy ointment before using this product because they
interfere with the ability of this product to adhere properly to the skin area
Keep out of reach of children. If swallowed, get medical help or contact a Poison
Control Center right away
Directions
• Adults: When practical, cleanse the affected area by patting or blotting with an
appropriate cleansing wipe. Gently dry by patting or blotting with toilet tissue or a soft
cloth before application of this product.
• For Pruritus Ani: Wait 20 seconds after drying. If the desire to scratch is gone, do not
apply cream. If the desire to scratch continues, apply a fingertip amount of ointment to
anal and perianal areas. Use finger cot or disposable gloves if desired. Rub or lightly
scratch (with fingertip, not fingernails) until the itch is satisfied.
• For Anal Fissures: Apply a fingertip amount of cream to the anal area and to the inner
rim of the anus.
• Children under 12 years of age: consult a doctor
• Apply externally to the affected area once or twice daily when pain or irritation from
anal fissures are present or ideally after bowel movement.
| PRANICURA
glycerin, kaolin, calamine, menthol cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - PRANICURA GROUP LLC (118320102) |
| Registrant - PRANICURA GROUP LLC (118320102) |