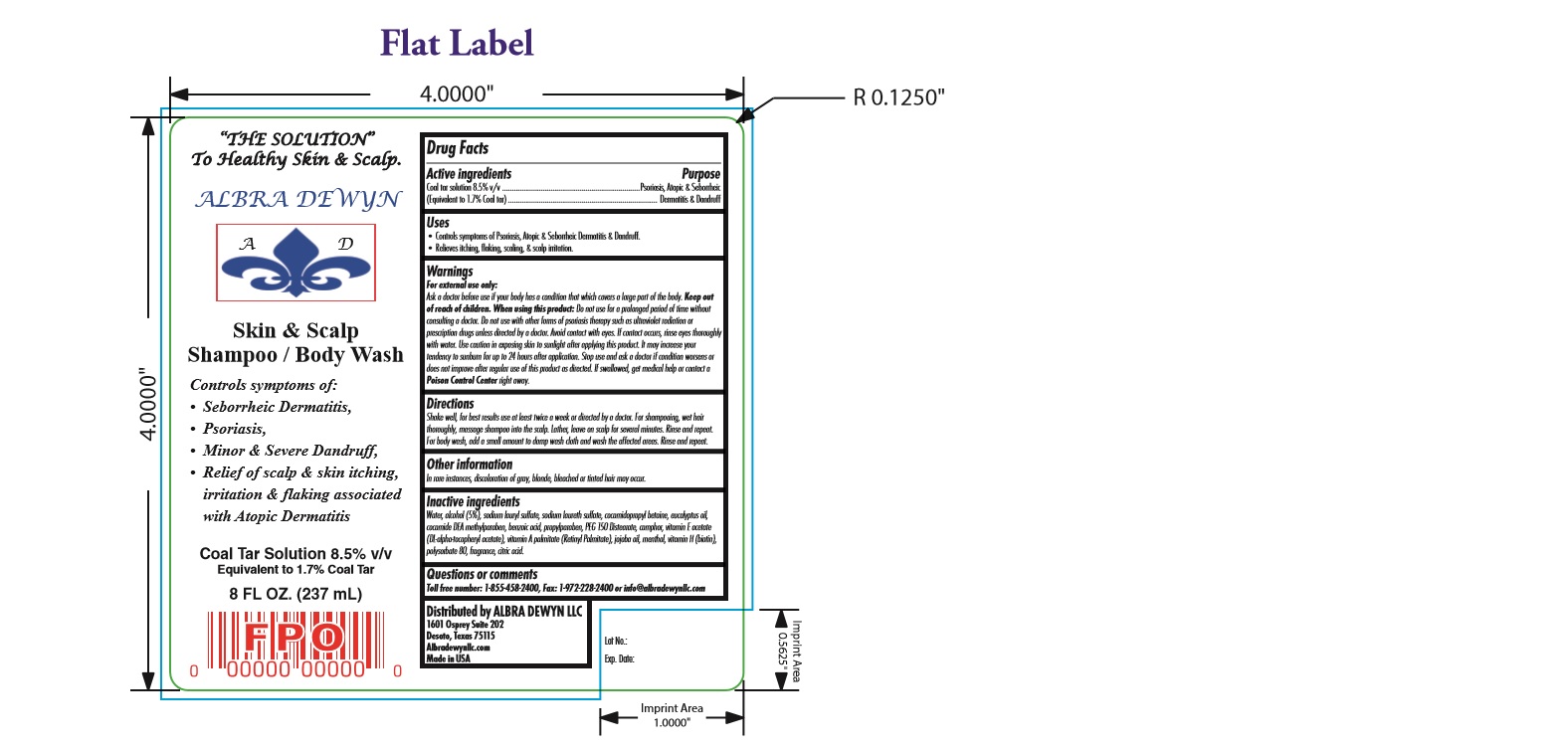
Label: SKIN AND SCALP- coal tar shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 59151-645-08 - Packager: Albra Dewyn LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 14, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- USES
-
WARNINGS
For external use only: Ask a doctor before use if your body has a condition that which covers a large part of the body. Keep out of reach of children. When using this product: Do not use for a prolonged period of time without consulting a doctor. Do not use with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed by a doctor. Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water. Use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application. Stop use and ask a doctor if condition worsens or does not improve after regular use of this product as directed. If swallowed, get medical help or contact a Poison Control Center right away. -
DIRECTIONS
Shake well, for best results use at least twice a week or directed by a doctor. For shampooing, wet hair thoroughly, massage shampoo into the scalp. Lather, leave on scalp for several minutes. Rinse and repeat. For body wash, add a small amount to damp wash cloth and wash the affected areas. Rinse and repeat
- PURPOSE
- OTHER INFORMATION
-
INactive
Inactive ingredients Water, alcohol (5%), sodium lauryl sulfate, sodium laureth sulfate, cocamidopropyl betaine, eucalyptus oil,
cocamide DEA methylparaben, benzoic acid, propylparaben, PEG 150 Distearate, camphor, vitamin E acetate (DL-alpha-tocopheryl acetate),
vitamin A palmitate (Retinyl Palmitate), jojoba oil, menthol, vitamin H (biotin), polysorbate 80, fragrance, citric acid. - questions or comments
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKIN AND SCALP
coal tar shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59151-645 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAL TAR (UNII: R533ESO2EC) (COAL TAR - UNII:R533ESO2EC) COAL TAR 17 mg in 1 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) COCAMIDOPROPYL DIMETHYLAMINE PROPIONATE (UNII: 83750MLC4A) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) CAMPHOR (NATURAL) (UNII: N20HL7Q941) JOJOBA OIL (UNII: 724GKU717M) BIOTIN (UNII: 6SO6U10H04) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) METHYLPARABEN (UNII: A2I8C7HI9T) BENZOIC ACID (UNII: 8SKN0B0MIM) PROPYLPARABEN (UNII: Z8IX2SC1OH) MENTHOL (UNII: L7T10EIP3A) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59151-645-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 09/01/2016 Labeler - Albra Dewyn LLC (069451336) Establishment Name Address ID/FEI Business Operations Albra Dewyn LLC 069451336 manufacture(59151-645)