Label: PRIMIDAR- lactobacillus and bifidobacterium capsule
- NHRIC Code(s): 0276-0700-30
- Packager: Misemer Pharmaceuticals, Inc.
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Primidar is an orally administered prescription probiotic formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
Primidar should be administered under the supervision of a licensed medical practitioner.
- HEALTH CLAIM
-
WARNINGS AND PRECAUTIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Primidar should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED:
- STORAGE:
-
PRINCIPAL DISPLAY PANEL
Primidar is an orally administered prescription probiotic supplement, indicated for the distinct nutritional requirements
of patients in need of dietary supplementation as determined by a licensed medical practitioner. Primidar should be
administered under the supervision of a licensed medical practitioner.
-
INGREDIENTS AND APPEARANCE
PRIMIDAR
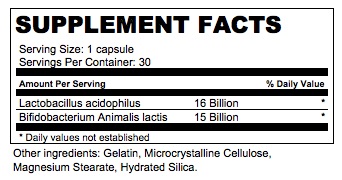
lactobacillus and bifidobacterium capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:0276-0700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTOBACILLUS ACIDOPHILUS (UNII: 1PRR1V42V5) (LACTOBACILLUS ACIDOPHILUS - UNII:1PRR1V42V5) LACTOBACILLUS ACIDOPHILUS 16000000000 [CFU] BIFIDOBACTERIUM ANIMALIS LACTIS (UNII: 5307V7XW8I) (BIFIDOBACTERIUM ANIMALIS LACTIS - UNII:5307V7XW8I) BIFIDOBACTERIUM ANIMALIS LACTIS 15000000000 [CFU] Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) HYDRATED SILICA (UNII: Y6O7T4G8P9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:0276-0700-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/01/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 16 mm scoring 1 Labeler - Misemer Pharmaceuticals, Inc. (784121365)