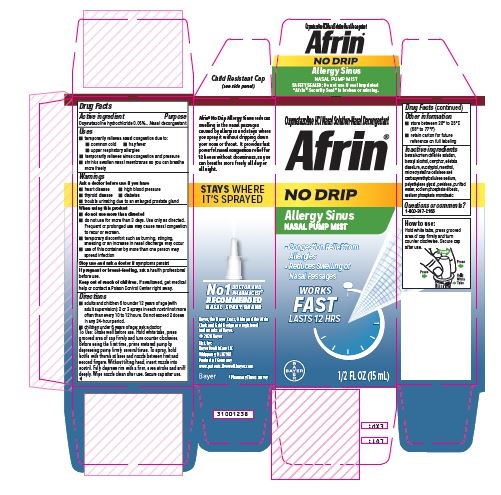
Label: AFRIN NO-DRIP ALLERGY SINUS- oxymetazoline hydrochloride spray, metered
- NDC Code(s): 11523-3220-1
- Packager: Bayer HealthCare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
-
WHEN USING
When using this product
● do not use more than directed
● do not use more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
● temporary discomfort may occur such as burning, stinging, sneezing or an increase in nasal discharge may occur
● use of this container by more than one person may spread infection
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Directions
· adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24 hour period.
· children under 6 years of age: ask a doctor
Shake well before use. Hold white tabs, press grooved area of cap firmly and turn counter clockwise. Before using the first time, prime metered pump by depressing pump firmly several times. To spray, hold bottle with thumb at base and nozzle between first and second fingers. Without tilting head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozzle clean after use. Secure cap after use.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- Questions or Comments
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AFRIN NO-DRIP ALLERGY SINUS
oxymetazoline hydrochloride spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-3220 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 mg in 1 mL Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CAMPHOR, (-)- (UNII: 213N3S8275) EDETATE DISODIUM (UNII: 7FLD91C86K) EUCALYPTOL (UNII: RV6J6604TK) MENTHOL (UNII: L7T10EIP3A) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) POVIDONE (UNII: FZ989GH94E) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-3220-1 1 in 1 CARTON 08/30/2016 1 15 mL in 1 BOTTLE, PUMP; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/30/2016 Labeler - Bayer HealthCare LLC (112117283)