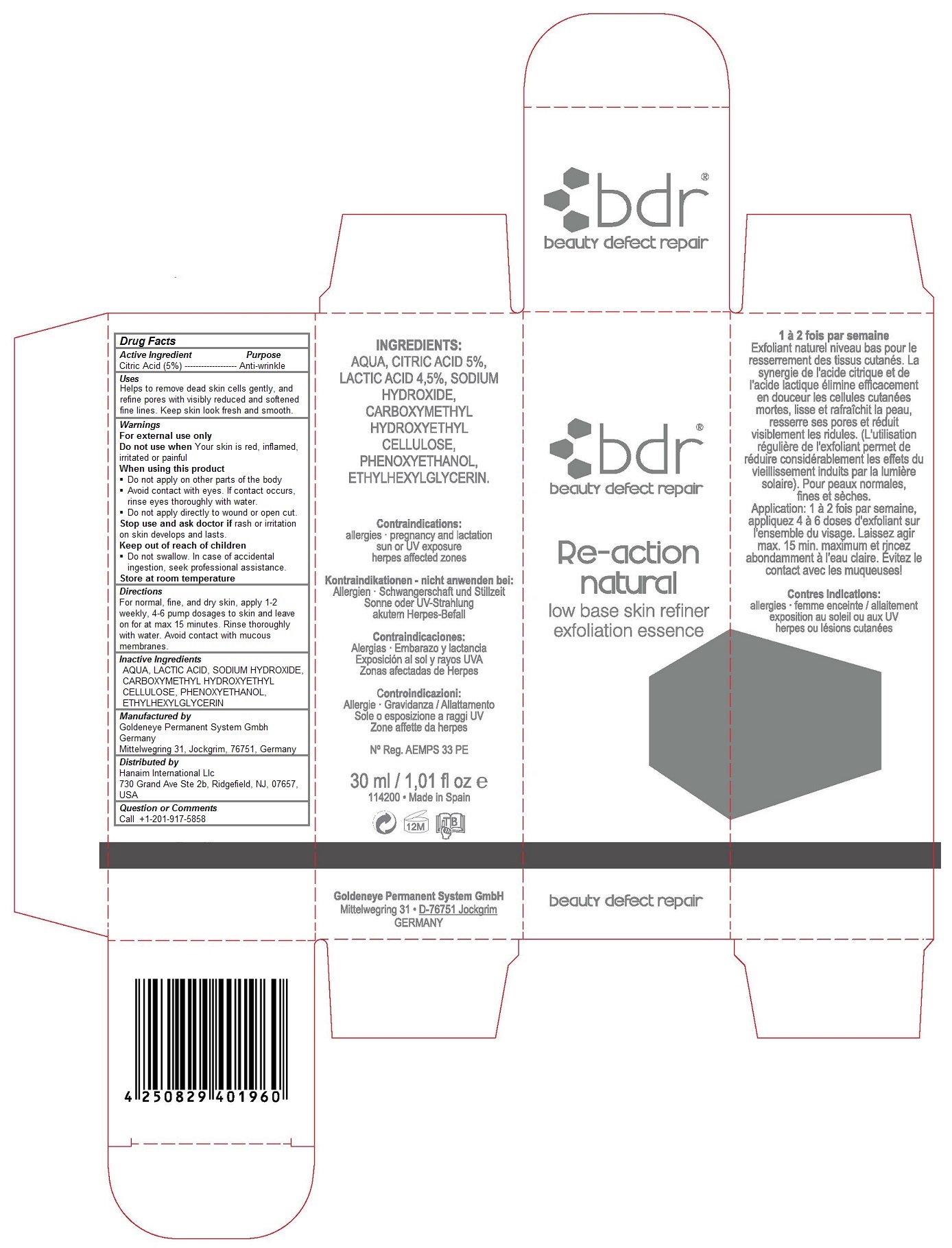
BDR RE-ACTION NATURAL LOW BASE SKIN REFINER EXFOLIATION ESSENCE- citric acid monohydrate liquid
Goldeneye Permanent System Gmbh Germany
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
GOLDENEYE - bdr Re-action natural low base skin refiner exfoliation essence
Keep out of reach of children
Do not swallow. In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Uses
Helps to refine pores with visibly reduced and softened fine lines. Keep skin look fresh and smooth.
Warnings
For external use only
Do not use when Your skin is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on skin develops and lasts.
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Store at room temperature
Directions
For normal, fine, and dry skin, apply 1-2 weekly, 4-6 pump dosages to skin and leave on for at max 15 minutes. Rinse thoroughly with water. Avoid contact with mucous membranes.
| BDR RE-ACTION NATURAL LOW BASE SKIN REFINER EXFOLIATION ESSENCE
citric acid monohydrate liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Goldeneye Permanent System Gmbh Germany (329178144) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Goldeneye Permanent System Gmbh Germany | 329178144 | manufacture(71056-102) | |