Label: ACQUA AROMA HAND SANITIZER GEL- alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 70848-410-30, 70848-413-80 - Packager: Smell It Industria e Comercio Ltda
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 6, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose:
- Use
- Warnings
- (For 1.0 FL OZ) Warnings:
- (For 12.9 FL OZ) Warnings:
- Warnings
- (For 1.0 FL OZ) Warnings
- (For 12.9 FL OZ) Warnings:
- Other Information:
- Directions
-
(For 1.0 FL OZ) Inactive Ingredients:
Inactive ingredients: Water (Aqua), Methyl Gluceth-20, Acrylates C10-30 Alkyl Acrylate Crosspolymer, Fragrance (Parfum), Aminomethyl Propanol, Mannitol, Cellulose, Citronellol, Linalool, Alpha-isomethyl Ionone, Coumarin, CI 77492, D-limonene, Geraniol, Tocopheryl Acetate, Hydroxypropyl Methylcellulose.
-
(For 12.9 FL OZ) Inactive Ingredients:
Inactive Ingredients: Water (Aqua), Acrylates C10-30 Alkyl Acrylate Crosspolymer, Methyl Gluceth-20, Fragrance (Parfum), Aminomethyl Propanol, Mannitol, Cellulose, Citronellol, Linalool, Alpha-isomethyl Ionone, Acrylates Copolymer, Coumarin, CI 77492, Hydroxypropylcellulose, Mica, CI 77891, D-limonene, Geraniol, Hydroxypropyl Methylcellulose, Triethyl Citrate, Acrylate Ammonium Methacrylate Copolymer, CI 77491, Caprylyl Capryl Glucoside, Caprylic Capric Triglyceride, Lauryl Glucoside, Talc, Tocopheryl Acetate.
- Questions
- (For 1.0 OZ) Primary Panel
- (For 12.9 OZ) Primary Panel
-
INGREDIENTS AND APPEARANCE
ACQUA AROMA HAND SANITIZER GEL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70848-410 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 21.024 mL in 30 mL Inactive Ingredients Ingredient Name Strength AMINOMETHYLPROPANOL (UNII: LU49E6626Q) 0.0315 mL in 30 mL POWDERED CELLULOSE (UNII: SMD1X3XO9M) 0.009 mL in 30 mL MANNITOL (UNII: 3OWL53L36A) 0.0138 mL in 30 mL .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.00024 mL in 30 mL WATER (UNII: 059QF0KO0R) 8.6655 mL in 30 mL CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) 0.09 mL in 30 mL METHYL GLUCETH-20 (UNII: J3QD0LD11P) 0.09 mL in 30 mL HYPROMELLOSES (UNII: 3NXW29V3WO) 0.00024 mL in 30 mL FERRIC OXIDE YELLOW (UNII: EX438O2MRT) 0.00072 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70848-410-30 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/01/2016 ACQUA AROMA HAND SANITIZER GEL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70848-413 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 266.304 mL in 380 mL Inactive Ingredients Ingredient Name Strength METHACRYLATE/METHOXY PEG-10 MALEATE/STYRENE COPOLYMER (UNII: 39DK5WQ2PR) 0.00304 mL in 380 mL ACRYLIC ACID/ISOPHORONE DIISOCYANATE/PEG-27 COPOLYMER (UNII: R0R8I3X29J) 0.02546 mL in 380 mL MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) 0.00304 mL in 380 mL TALC (UNII: 7SEV7J4R1U) 0.00304 mL in 380 mL AMINOMETHYLPROPANOL (UNII: LU49E6626Q) 0.399 mL in 380 mL POWDERED CELLULOSE (UNII: SMD1X3XO9M) 0.1824 mL in 380 mL MANNITOL (UNII: 3OWL53L36A) 0.361 mL in 380 mL .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.00304 mL in 380 mL WATER (UNII: 059QF0KO0R) 109.421 mL in 380 mL CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) 1.14 mL in 380 mL METHYL GLUCETH-20 (UNII: J3QD0LD11P) 1.14 mL in 380 mL HYPROMELLOSES (UNII: 3NXW29V3WO) 0.00608 mL in 380 mL FERRIC OXIDE YELLOW (UNII: EX438O2MRT) 0.01216 mL in 380 mL C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) 0.00304 mL in 380 mL FERRIC OXIDE RED (UNII: 1K09F3G675) 0.00304 mL in 380 mL HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) 0.01216 mL in 380 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.01026 mL in 380 mL LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) 0.00304 mL in 380 mL MICA (UNII: V8A1AW0880) 0.01216 mL in 380 mL TRIETHYL CITRATE (UNII: 8Z96QXD6UM) 0.00304 mL in 380 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70848-413-80 380 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/01/2016 Labeler - Smell It Industria e Comercio Ltda (678349245) Registrant - Acqua Aroma US LLC (080193028) Establishment Name Address ID/FEI Business Operations Smell It Industria e Comercio Ltda 678349245 manufacture(70848-410, 70848-413)


 bacteria on hands.
bacteria on hands.
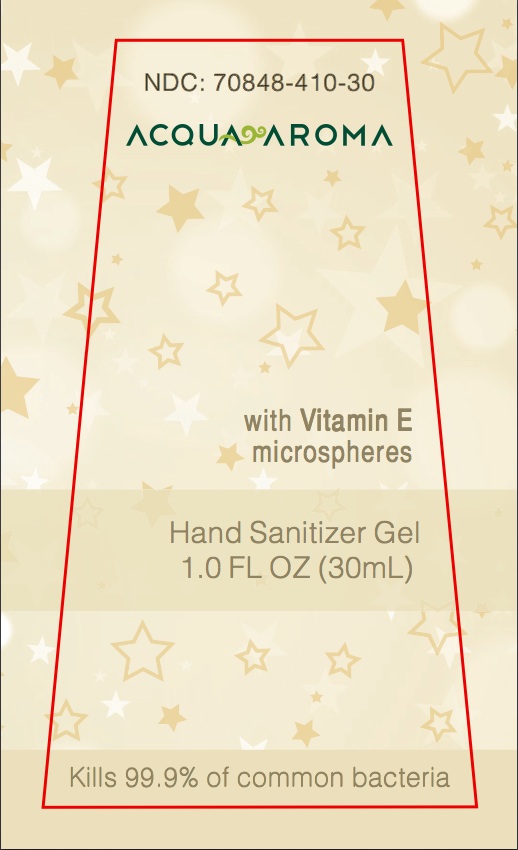
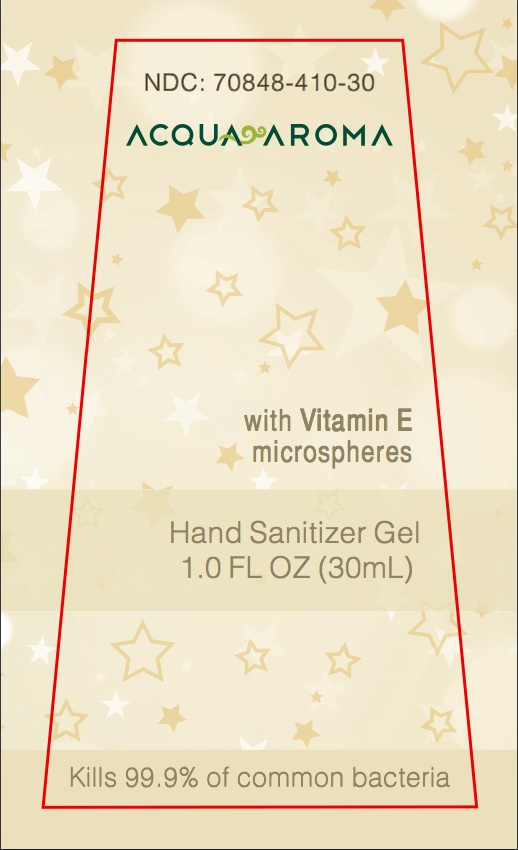 NDC Number Acqua Aroma With Vitamin E microspheres Hand Sanitizer Gel 1.0 FL OZ (30ML) Kills 99.9% of common bacteria
NDC Number Acqua Aroma With Vitamin E microspheres Hand Sanitizer Gel 1.0 FL OZ (30ML) Kills 99.9% of common bacteria
 Acqua Aroma The Scent of Your Home NDC Number With Vitamin E microspheres Hand Sanitizer Gel 12.9 FL OZ (380ML) Kills 99.9% of common bacteria
Acqua Aroma The Scent of Your Home NDC Number With Vitamin E microspheres Hand Sanitizer Gel 12.9 FL OZ (380ML) Kills 99.9% of common bacteria