Label: FLUDARABINE PHOSPHATE injection, powder, lyophilized, for solution
- NDC Code(s): 45963-609-55
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING:
Fludarabine Phosphate for Injection should be administered under the supervision of a qualified physician experienced in the use of antineoplastic therapy. Fludarabine Phosphate for Injection can severely suppress bone marrow function. When used at high doses in dose-ranging studies in patients with acute leukemia, Fludarabine Phosphate for Injection was associated with severe neurologic effects, including blindness, coma, and death. This severe central nervous system toxicity occurred in 36% of patients treated with doses approximately four times greater (96 mg/m2/day for 5 to 7 days) than the recommended dose. Similar severe central nervous system toxicity, including coma, seizures, agitation and confusion, has been reported in patients treated at doses in the range of the dose recommended for chronic lymphocytic leukemia.
Instances of life-threatening and sometimes fatal autoimmune phenomena such as hemolytic anemia, autoimmune thrombocytopenia/thrombocytopenic purpura (ITP), Evans syndrome, and acquired hemophilia have been reported to occur after one or more cycles of treatment with Fludarabine Phosphate for Injection. Patients undergoing treatment with Fludarabine Phosphate for Injection should be evaluated and closely monitored for hemolysis.
In a clinical investigation using Fludarabine Phosphate for Injection in combination with pentostatin (deoxycoformycin) for the treatment of refractory chronic lymphocytic leukemia (CLL), there was an unacceptably high incidence of fatal pulmonary toxicity. Therefore, the use of Fludarabine Phosphate for Injection in combination with pentostatin is not recommended.
-
DESCRIPTION
Fludarabine Phosphate for Injection, USP contains fludarabine phosphate USP, a fluorinated nucleotide analog of the antiviral agent vidarabine, 9-β-D-arabinofuranosyladenine (ara-A) that is relatively resistant to deamination by adenosine deaminase. Each vial of sterile lyophilized solid cake contains 50 mg of the active ingredient fludarabine phosphate, 50 mg of mannitol, and sodium hydroxide to adjust pH to 7.7. The pH range for the final product is 7.2 to 8.2. Reconstitution with 2 mL of Sterile Water for Injection, USP, results in a solution containing 25 mg/mL of fludarabine phosphate intended for intravenous administration.
The chemical name for fludarabine phosphate is 9H-Purin-6-amine, 2-fluoro-9-(5-0-phosphono-β-D-arabino-furanosyl) (2-fluoro-ara-AMP). The molecular formula of fludarabine phosphate is C10H13FN5O7P (MW 365.2) and the structure is:
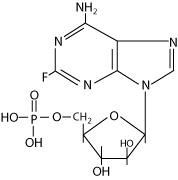
-
CLINICAL PHARMACOLOGY
Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite appears to act by inhibiting DNA polymerase alpha, ribonucleotide reductase and DNA primase, thus inhibiting DNA synthesis. The mechanism of action of this antimetabolite is not completely characterized and may be multi-faceted.
Phase I studies in humans have demonstrated that fludarabine phosphate is rapidly converted to the active metabolite, 2-fluoro-ara-A, within minutes after intravenous infusion. Consequently, clinical pharmacology studies have focused on 2-fluoro-ara-A pharmacokinetics. After the five daily doses of
25 mg 2-fluoro-ara-AMP/m2 to cancer patients infused over 30 minutes, 2-fluoro-ara-A concentrations show a moderate accumulation. During a 5-day treatment schedule, 2-fluoro-ara-A plasma trough levels increased by a factor of about 2. The terminal half-life of 2-fluoro-ara-A was estimated as approximately 20 hours. In vitro, plasma protein binding of fludarabine ranged between 19% and 29%.A correlation was noted between the degree of absolute granulocyte count nadir and increased area under the concentration x time curve (AUC).
Special Populations
Pediatric Patients
Limited pharmacokinetic data for Fludarabine Phosphate for Injection are available from a published study of children (ages 1 to 21 years) with refractory acute leukemias or solid tumors (Children's Cancer Group Study 0971). When Fludarabine Phosphate for Injection was administered as a loading dose over 10 minutes immediately followed by a 5-day continuous infusion, steady-state conditions were reached early.
Patients with Renal Impairment
The total body clearance of the principal metabolite 2-fluoro-ara-A correlated with the creatinine clearance, indicating the importance of the renal excretion pathway for the elimination of the drug. Renal clearance represents approximately 40% of the total body clearance. Patients with creatinine clearance 30 to 79 mL/min should have their Fludarabine Phosphate for Injection dose reduced and be monitored closely for excessive toxicity. Due to insufficient data, Fludarabine Phosphate for Injection should not be administered to patients with creatinine clearance less than 30 mL/min (see DOSAGE AND ADMINISTRATION section).
-
CLINICAL STUDIES
Two single-arm, open-label studies of Fludarabine Phosphate for Injection have been conducted in adult patients with CLL refractory to at least one prior standard alkylating-agent containing regimen. In a study conducted by M.D. Anderson Cancer Center (MDAH), 48 patients were treated with a dose of 22 to 40 mg/m2 daily for 5 days every 28 days. Another study conducted by the Southwest Oncology Group (SWOG) involved 31 patients treated with a dose of 15 to 25 mg/m2 daily for 5 days every 28 days. The overall objective response rates were 48% and 32% in the MDAH and SWOG studies, respectively. The complete response rate in both studies was 13%; the partial response rate was 35% in the MDAH study and 19% in the SWOG study. These response rates were obtained using standardized response criteria developed by the National Cancer Institute CLL Working Group and were achieved in heavily pretreated patients. The ability of Fludarabine Phosphate for Injection to induce a significant rate of response in refractory patients suggests minimal cross-resistance with commonly used anti-CLL agents.
The median time to response in the MDAH and SWOG studies was 7 weeks (range of 1 to 68 weeks) and 21 weeks (range of 1 to 53 weeks), respectively. The median duration of disease control was 91 weeks (MDAH) and 65 weeks (SWOG). The median survival of all refractory CLL patients treated with Fludarabine Phosphate for Injection was 43 weeks and 52 weeks in the MDAH and SWOG studies, respectively.
Rai stage improved to Stage II or better in 7 of 12 MDAH responders (58%) and in 5 of 7 SWOG responders (71%) who were Stage III or IV at baseline. In the combined studies, mean hemoglobin concentration improved from 9 g/dL at baseline to 11.8 g/dL at the time of response in a subgroup of anemic patients. Similarly, average platelet count improved from 63,500/mm3 to 103,300/mm3 at the time of response in a subgroup of patients who were thrombocytopenic at baseline.
-
INDICATIONS AND USAGE
Fludarabine Phosphate for Injection is indicated for the treatment of adult patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to or whose disease has progressed during treatment with at least one standard alkylating-agent containing regimen. The safety and effectiveness of Fludarabine Phosphate for Injection in previously untreated or non-refractory patients with CLL have not been established.
- CONTRAINDICATIONS
-
WARNINGS
(See BOXED WARNINGS)
Dose Dependent Neurologic Toxicities
There are clear dose-dependent toxic effects seen with Fludarabine Phosphate for Injection. Dose levels approximately 4 times greater (96 mg/m2/day for 5 to 7 days) than that recommended for CLL (25 mg/m2/day for 5 days) were associated with a syndrome characterized by delayed blindness, coma and death. Symptoms appeared from 21 to 60 days following the last dose. Thirteen of 36 patients (36%) who received Fludarabine Phosphate for Injection at high doses (96 mg/m2/day for 5 to 7 days) developed this severe neurotoxicity. Similar severe central nervous system toxicity, including coma, seizures, agitation and confusion, has been reported in patients treated at doses in the range of the dose recommended for chronic lymphocytic leukemia.
In post-marketing experience neurotoxicity has been reported to occur either earlier or later than in clinical trials (range 7 to 225 days).
The effect of chronic administration of Fludarabine Phosphate for Injection on the central nervous system is unknown; however, patients have received the recommended dose for up to 15 courses of therapy.
Bone Marrow Suppression
Severe bone marrow suppression, notably anemia, thrombocytopenia and neutropenia, has been reported in patients treated with Fludarabine Phosphate for Injection. In a Phase I study in adult solid tumor patients, the median time to nadir counts was 13 days (range, 3 to 25 days) for granulocytes and 16 days (range, 2 to 32) for platelets. Most patients had hematologic impairment at baseline either as a result of disease or as a result of prior myelosuppressive therapy. Cumulative myelosuppression may be seen. While chemotherapy-induced myelosuppression is often reversible, administration of Fludarabine Phosphate for Injection requires careful hematologic monitoring.
Several instances of trilineage bone marrow hypoplasia or aplasia resulting in pancytopenia, sometimes resulting in death, have been reported in adult patients. The duration of clinically significant cytopenia in the reported cases has ranged from approximately 2 months to approximately 1 year. These episodes have occurred both in previously treated or untreated patients.
Autoimmune Reactions
Instances of life-threatening and sometimes fatal autoimmune phenomena such as hemolytic anemia, autoimmune thrombocytopenia/thrombocytopenic purpura (ITP), Evans syndrome, and acquired hemophilia have been reported to occur after one or more cycles of treatment with Fludarabine Phosphate for Injection in patients with or without a previous history of autoimmune hemolytic anemia or a positive Coombs' test and who may or may not be in remission from their disease. Steroids may or may not be effective in controlling these hemolytic episodes. The majority of patients rechallenged with Fludarabine Phosphate for Injection developed a recurrence in the hemolytic process. The mechanism(s) which predispose patients to the development of this complication has not been identified. Patients undergoing treatment with Fludarabine Phosphate for Injection should be evaluated and closely monitored for hemolysis. Discontinuation of therapy with Fludarabine Phosphate for Injection is recommended in case of hemolysis.
Transfusion Associated Graft-Versus-Host Disease
Transfusion-associated graft-versus-host disease has been observed after transfusion of non-irradiated blood in Fludarabine Phosphate for Injection treated patients. Fatal outcome as a consequence of this disease has been reported. Therefore, to minimize the risk of transfusion-associated graft-versus-host disease, patients who require blood transfusion and who are undergoing, or who have received, treatment with Fludarabine Phosphate for Injection should receive irradiated blood only.
Pulmonary Toxicity
In a clinical investigation using Fludarabine Phosphate for Injection in combination with pentostatin (deoxycoformycin) for the treatment of refractory chronic lymphocytic leukemia (CLL) in adults, there was an unacceptably high incidence of fatal pulmonary toxicity. Therefore, the use of Fludarabine Phosphate for Injection in combination with pentostatin is not recommended.
Pregnancy Category D
Based on its mechanism of action, fludarabine phosphate can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Fludarabine Phosphate for Injection in pregnant women. Fludarabine administered to rats and rabbits during organogenesis caused an increase in resorptions, skeletal and visceral malformations and decreased fetal body weights. If Fludarabine Phosphate for Injection is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Male Fertility and Reproductive Outcomes
Males with female sexual partners of childbearing potential should use contraception during and after cessation of Fludarabine Phosphate for Injection therapy. Fludarabine may damage testicular tissue and spermatozoa. Possible sperm DNA damage raises concerns about loss of fertility and genetic abnormalities in fetuses. The duration of this effect is uncertain. (See PRECAUTIONS, Impairment of Fertility)
-
PRECAUTIONS
General
Fludarabine Phosphate for Injection is a potent antineoplastic agent with potentially significant toxic side effects. Patients undergoing therapy should be closely observed for signs of hematologic and nonhematologic toxicity. Periodic assessment of peripheral blood counts is recommended to detect the development of anemia, neutropenia and thrombocytopenia.
In patients with impaired state of health, Fludarabine Phosphate for Injection should be given with caution and after careful risk/benefit consideration. This applies especially for patients with severe impairment of bone marrow function (thrombocytopenia, anemia, and/or granulocytopenia), immunodeficiency or with a history of opportunistic infection. Prophylactic treatment should be considered in patients at increased risk of developing opportunistic infections.
Fludarabine Phosphate for Injection may reduce the ability to drive or use machines, since fatigue, weakness, visual disturbances, confusion, agitation and seizures have been observed.
Tumor Cell Lysis
Tumor lysis syndrome has been associated with Fludarabine Phosphate for Injection treatment. This syndrome has been reported in CLL patients with large tumor burden. Since Fludarabine Phosphate for Injection can induce a response as early as the first week of treatment, precautions should be taken in those patients at risk of developing this complication.
Renal Impairment
Fludarabine Phosphate for Injection must be administered cautiously in patients with renal impairment. The total body clearance of 2-fluoro-ara-A has been shown to be directly correlated with creatinine clearance. Patients with creatinine clearance 30 to 79 mL/min should have their Fludarabine Phosphate for Injection dose reduced and be monitored closely for excessive toxicity. Fludarabine Phosphate for Injection should not be administered to patients with creatinine clearance less than 30 mL/min. (See DOSAGE AND ADMINISTRATION section).
In patients aged 65 years or older, creatinine clearance should be measured before start of treatment.
Laboratory Tests
During treatment, the patient's hematologic profile (particularly neutrophils and platelets) should be monitored regularly to determine the degree of hematopoietic suppression.
Drug Interactions
The use of Fludarabine Phosphate for Injection in combination with pentostatin is not recommended due to the risk of fatal pulmonary toxicity (see WARNINGS section).
Carcinogenesis
No animal carcinogenicity studies with Fludarabine Phosphate for Injection have been conducted.
Mutagenesis
Fludarabine phosphate was not mutagenic to bacteria (Ames test) or mammalian cells (HGRPT assay in Chinese hamster ovary cells) either in the presence or absence of metabolic activation. Fludarabine phosphate was clastogenic in vitro to Chinese hamster ovary cells (chromosome aberrations in the presence of metabolic activation) and induced sister chromatid exchanges both with and without metabolic activation. In addition, fludarabine phosphate was clastogenic in vivo (mouse micronucleus assay) but was not mutagenic to germ cells (dominant lethal test in male mice).
Impairment of Fertility
Studies in mice, rats and dogs have demonstrated dose-related adverse effects on the male reproductive system. Observations consisted of a decrease in mean testicular weights in mice and rats with a trend toward decreased testicular weights in dogs and degeneration and necrosis of spermatogenic epithelium of the testes in mice, rats and dogs. The possible adverse effects on fertility in humans have not been adequately evaluated.
Pregnancy
(See WARNINGS section).
Based on its mechanism of action, fludarabine phosphate can cause fetal harm when administered to a pregnant woman. There are not adequate and well-controlled studies of fludarabine phosphate in pregnant women. Fludarabine phosphate was embryolethal and teratogenic in rats and rabbits. If Fludarabine Phosphate for Injection is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
In rats, repeated intravenous doses of fludarabine phosphate at 2.4 times and 7.2 times the recommended human IV dose (25 mg/m2) administered during organogenesis caused an increase in resorptions, skeletal and visceral malformations (cleft palate, exencephaly, and fetal vertebrae deformities) and decreased fetal body weights. Maternal toxicity was not apparent at 2.4 times the human IV dose, and was limited to slight body weight decreases at 7.2 times the human IV dose. In rabbits, repeated intravenous doses of fludarabine phosphate at 3.8 times the human IV dose administered during organogenesis increased embryo and fetal lethality as indicated by increased resorptions and a decrease in live fetuses. A significant increase in malformations including cleft palate, hydrocephaly, adactyly, brachydactyly, fusions of the digits, diaphragmatic hernia, heart/great vessel defects, and vertebrae/rib anomalies were seen in all dose levels (≥0.5 times the human IV dose).
Nursing Mothers
It is not known whether fludarabine phosphate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions including tumorgenicity in nursing infants, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Data submitted to the FDA was insufficient to establish efficacy in any childhood malignancy. Fludarabine Phosphate for Injection was evaluated in 62 pediatric patients (median age 10, range 1 to 21) with refractory acute leukemia (45 patients) or solid tumors (17 patients). The Fludarabine Phosphate for Injection regimen tested for pediatric acute lymphocytic leukemia (ALL) patients was a loading bolus of 10.5 mg/m2/day followed by a continuous infusion of 30.5 mg/m2/day for 5 days. In 12 pediatric patients with solid tumors, dose-limiting myelosuppression was observed with a loading dose of 8 mg/m2/day followed by a continuous infusion of 23.5 mg/m2/day for 5 days. The maximum tolerated dose was a loading dose of 7 mg/m2/day followed by a continuous infusion of 20 mg/m2/day for 5 days. Treatment toxicity included bone marrow suppression. Platelet counts appeared to be more sensitive to the effects of Fludarabine Phosphate for Injection than hemoglobin and white blood cell counts. Other adverse events included fever, chills, asthenia, rash, nausea, vomiting, diarrhea, and infection. There were no reported occurrences of peripheral neuropathy or pulmonary hypersensitivity reaction.
-
ADVERSE REACTIONS
Very common adverse events include myelosuppression (neutropenia, thrombocytopenia and anemia), fever and chills, fatigue, weakness, infection, pneumonia, cough, nausea, vomiting, and diarrhea. Other commonly reported events include malaise, mucositis and anorexia. Serious opportunistic infections (such as latent viral reactivation, herpes zoster virus, Epstein-Barr virus, and progressive multifocal leukoencephalopathy) have occurred in CLL patients treated with Fludarabine Phosphate for Injection. Adverse events and those reactions which are more clearly related to the drug are arranged below according to body system.
Hematopoietic Systems
Hematologic events (neutropenia, thrombocytopenia, and/or anemia) were reported in the majority of CLL patients treated with Fludarabine Phosphate for Injection. During Fludarabine Phosphate for Injection treatment of 133 patients with CLL, the absolute neutrophil count decreased to less than 500/mm3 in 59% of patients, hemoglobin decreased from pretreatment values by at least 2 grams percent in 60%, and platelet count decreased from pretreatment values by at least 50% in 55%. Myelosuppression may be severe, cumulative, and may affect multiple cell lines. Bone marrow fibrosis occurred in one CLL patient treated with Fludarabine Phosphate for Injection.
Several instances of trilineage bone marrow hypoplasia or aplasia resulting in pancytopenia, sometimes resulting in death, have been reported in post marketing surveillance. The duration of clinically significant cytopenia in the reported cases has ranged from approximately 2 months to approximately 1 year. These episodes have occurred both in previously treated or untreated patients.
Life-threatening and sometimes fatal autoimmune phenomena such as hemolytic anemia, autoimmune thrombocytopenia/thrombocytopenic purpura (ITP), Evans syndrome, and acquired hemophilia have been reported to occur in patients receiving Fludarabine Phosphate for Injection (see WARNINGS section). The majority of patients rechallenged with Fludarabine Phosphate for Injection developed a recurrence in the hemolytic process.
In postmarketing experience, cases of myelodysplastic syndrome and acute myeloid leukemia, mainly associated with prior, concomitant or subsequent treatment with alkylating agents, topoisomerase inhibitors, or irradiation have been reported.
Infections
Serious and sometimes fatal infections, including opportunistic infections and reactivations of latent viral infections such as VZV (herpes zoster), Epstein-Barr virus and JC virus (progressive multifocal leukoencephalopathy) have been reported in patients treated with Fludarabine Phosphate for Injection.
Rare cases of Epstein Barr virus (EBV) associated lymphoproliferative disorders have been reported in patients treated with Fludarabine Phosphate for Injection.
In postmarketing experience, cases of progressive multifocal leukoencephalopathy have been reported. Most cases had a fatal outcome. Many of these cases were confounded by prior and/or concurrent chemotherapy. The time to onset has ranged from a few weeks to approximately one year after initiating treatment.
Of the 133 adult CLL patients in the two trials, there were 29 fatalities during study, approximately 50% of which were due to infection.
Metabolic
Tumor lysis syndrome has been reported in CLL patients treated with Fludarabine Phosphate for Injection. This complication may include hyperuricemia, hyperphosphatemia, hypocalcemia, metabolic acidosis, hyperkalemia, hematuria, urate crystalluria, and renal failure. The onset of this syndrome may be heralded by flank pain and hematuria.
Nervous System (see WARNINGS section)
Objective weakness, agitation, confusion, seizures, visual disturbances, optic neuritis, optic neuropathy, blindness and coma have occurred in CLL patients treated with Fludarabine Phosphate for Injection at the recommended dose. Peripheral neuropathy has been observed in patients treated with Fludarabine Phosphate for Injection and one case of wrist-drop was reported. There have been additional reports of cerebral hemorrhage though the frequency is not known.
Pulmonary System
Pneumonia, a frequent manifestation of infection in CLL patients, occurred in 16% and 22% of those treated with Fludarabine Phosphate for Injection in the MDAH and SWOG studies, respectively. Pulmonary hypersensitivity reactions to Fludarabine Phosphate for Injection characterized by dyspnea, cough and interstitial pulmonary infiltrate have been observed.
In post-marketing experience, cases of severe pulmonary toxicity have been observed with Fludarabine Phosphate for Injection use which resulted in ARDS, respiratory distress, pulmonary hemorrhage, pulmonary fibrosis, pneumonitis and respiratory failure. After an infectious origin has been excluded, some patients experienced symptom improvement with corticosteroids.
Gastrointestinal System
Gastrointestinal disturbances such as nausea and vomiting, anorexia, diarrhea, stomatitis and gastrointestinal bleeding and hemorrhage have been reported in patients treated with Fludarabine Phosphate for Injection. Elevations of pancreatic enzyme levels have also been reported.
Cardiovascular
Edema has been frequently reported. One patient developed a pericardial effusion possibly related to treatment with Fludarabine Phosphate for Injection. There have been additional reports of heart failure and arrhythmia though the frequency is rare. No other severe cardiovascular events were considered to be drug related.
Genitourinary System
Rare cases of hemorrhagic cystitis have been reported in patients treated with Fludarabine Phosphate for Injection.
Skin
Skin toxicity, consisting primarily of skin rashes, has been reported in patients treated with Fludarabine Phosphate for Injection. Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis and pemphigus have been reported, with fatal outcomes in some cases.
Neoplasms
Worsening or flare-up of pre-existing skin cancer lesions, as well as new onset of skin cancer, has been reported in patients during or after treatment with Fludarabine Phosphate for Injection.
Hepatobiliary Disorders
Elevations of hepatic enzyme levels have been reported.
Data in the following table are derived from the 133 patients with CLL who received Fludarabine Phosphate for Injection in the MDAH and SWOG studies.
PERCENT OF CLL PATIENTS REPORTING NONHEMATOLOGIC ADVERSE EVENTS ADVERSE EVENTS
MDAH (N=101)
SWOG (N=32)
ANY ADVERSE EVENT
88%
91%
BODY AS A WHOLE
72
84
FEVER
60
69
CHILLS
11
19
FATIGUE
10
38
INFECTION
33
44
PAIN
20
22
MALAISE
8
6
DIAPHORESIS
1
13
ALOPECIA
0
3
ANAPHYLAXIS
1
0
HEMORRHAGE
1
0
HYPERGLYCEMIA
1
6
DEHYDRATION
1
0
NEUROLOGICAL
21
69
WEAKNESS
9
65
PARESTHESIA
4
12
HEADACHE
3
0
VISUAL DISTURBANCE
3
15
HEARING LOSS
2
6
SLEEP DISORDER
1
3
DEPRESSION
1
0
CEREBELLAR SYNDROME
1
0
IMPAIRED MENTATION
1
0
PULMONARY
35
69
COUGH
10
44
PNEUMONIA
16
22
DYSPNEA
9
22
SINUSITIS
5
0
PHARYNGITIS
0
9
UPPER RESPIRATORY INFECTION
2
16
ALLERGIC PNEUMONITIS
0
6
EPISTAXIS
1
0
HEMOPTYSIS
1
6
BRONCHITIS
1
0
HYPOXIA
1
0
GASTROINTESTINAL
46
63
NAUSEA/VOMITING
36
31
DIARRHEA
15
13
ANOREXIA
7
34
STOMATITIS
9
0
GI BLEEDING
3
13
ESOPHAGITIS
3
0
MUCOSITIS
2
0
LIVER FAILURE
1
0
ABNORMAL LIVER FUNCTION TEST
1
3
CHOLELITHIASIS
0
3
CONSTIPATION
1
3
DYSPHAGIA
1
0
CUTANEOUS
17
18
RASH
15
15
PRURITUS
1
3
SEBORRHEA
1
0
GENITOURINARY
12
22
DYSURIA
4
3
URINARY INFECTION
2
15
HEMATURIA
2
3
RENAL FAILURE
1
0
ABNORMAL RENAL FUNCTION TEST
1
0
PROTEINURIA
1
0
HESITANCY
0
3
CARDIOVASCULAR
12
38
EDEMA
8
19
ANGINA
0
6
CONGESTIVE HEART FAILURE
0
3
ARRHYTHMIA
0
3
SUPRAVENTRICULAR TACHYCARDIA
0
3
MYOCARDIAL INFARCTION
0
3
DEEP VENOUS THROMBOSIS
1
3
PHLEBITIS
1
3
TRANSIENT ISCHEMIC ATTACK
1
0
ANEURYSM
1
0
CEREBROVASCULAR ACCIDENT
0
3
MUSCULOSKELETAL
7
16
MYALGIA
4
16
OSTEOPOROSIS
2
0
ARTHRALGIA
1
0
TUMOR LYSIS SYNDROME
1
0
More than 3000 adult patients received Fludarabine Phosphate for Injection in studies of other leukemias, lymphomas, and other solid tumors. The spectrum of adverse effects reported in these studies was consistent with the data presented above.
To report SUSPECTED ADVERSE EVENTS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch for voluntary reporting of adverse reactions.
-
OVERDOSAGE
High doses of Fludarabine Phosphate for Injection (see WARNINGS section) have been associated with an irreversible central nervous system toxicity characterized by delayed blindness, coma and death. High doses are also associated with severe thrombocytopenia and neutropenia due to bone marrow suppression. There is no known specific antidote for Fludarabine Phosphate for Injection overdosage. Treatment consists of drug discontinuation and supportive therapy.
-
DOSAGE AND ADMINISTRATION
Usual Dose
The recommended adult dose of Fludarabine Phosphate for Injection is 25 mg/m2 administered intravenously over a period of approximately 30 minutes daily for five consecutive days. Each 5 day course of treatment should commence every 28 days. Dosage may be decreased or delayed based on evidence of hematologic or nonhematologic toxicity. Physicians should consider delaying or discontinuing the drug if neurotoxicity occurs.
A number of clinical settings may predispose to increased toxicity from Fludarabine Phosphate for Injection. These include advanced age, renal impairment, and bone marrow impairment. Such patients should be monitored closely for excessive toxicity and the dose modified accordingly.
The optimal duration of treatment has not been clearly established. It is recommended that three additional cycles of Fludarabine Phosphate for Injection be administered following the achievement of a maximal response and then the drug should be discontinued.
Renal Impairment
Adjustments to the starting dose are recommended to provide appropriate drug exposure in patients with creatinine clearance 30 to 79 mL/min, as estimated by the Cockroft-Gault equations. These adjustments are based on a pharmacokinetic study in patients with renal impairment. Fludarabine Phosphate for Injection should not be administered to patients with creatinine clearance less than 30 mL/min.
Starting Dose Adjustment for Renal Impairment
Creatinine Clearance Starting Dose ≥ 80 mL/min 25 mg/m2 (full dose) 50 to 79 mL/min 20 mg/m2 30 to 49 mL/min 15 mg/m2 < 30 mL/min do not administer Renally impaired patients should be monitored closely for excessive toxicity and the dose modified accordingly.
Preparation of Solutions
Fludarabine Phosphate for Injection should be prepared for parenteral use by aseptically adding Sterile Water for Injection, USP. When reconstituted with 2 mL of Sterile Water for Injection, the solid cake should fully dissolve in 15 seconds or less; each mL of the resulting solution will contain 25 mg of fludarabine phosphate, 25 mg of mannitol, and sodium hydroxide to adjust the pH to 7.7. The pH range for the final product is 7.2 to 8.2. In clinical studies, the product has been diluted in 100 cc or 125 cc of 5% Dextrose Injection, USP, or 0.9% Sodium Chloride, USP.
Reconstituted Fludarabine Phosphate for Injection contains no antimicrobial preservative and thus should be used within 8 hours of reconstitution. Care must be taken to assure the sterility of prepared solutions. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Fludarabine Phosphate for Injection should not be mixed with other drugs.
Handling and Disposal
Procedures for proper handling and disposal should be considered. Consideration should be given to handling and disposal according to guidelines issued for cytotoxic drugs. Several guidelines on this subject have been published.1-4.
Caution should be exercised in the handling and preparation of Fludarabine Phosphate for Injection solution. The use of latex gloves and safety glasses is recommended to avoid exposure in case of breakage of the vial or other accidental spillage. If the solution contacts the skin or mucous membranes, wash thoroughly with soap and water; rinse eyes thoroughly with plain water. Avoid exposure by inhalation or by direct contact of the skin or mucous membranes.
-
HOW SUPPLIED
Fludarabine Phosphate for Injection, USP is a white, lyophilized solid cake. Each single-dose vial contains 50 mg of fludarabine phosphate USP, 50 mg of mannitol, and sodium hydroxide to adjust pH to 7.7. The pH range for the final product is 7.2 to 8.2.
Fludarabine Phosphate for Injection, USP, NDC 45963-609-55, is supplied in a clear glass single-dose vial and packaged in a carton.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F), in the original package.
The container closure is not made with natural rubber latex.
Sterile, Nonpyrogenic, Preservative-free
-
REFERENCES
- Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. NIOSH Alert 2004-165.
- OSHA Technical Manual, TED 1-0. 15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006: 63: 1172-1193.
- Polvich, M., White, J.M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd ed.). Pittsburgh, PA: Oncology Nursing Society
Manufactured In Romania By:
Sindan Pharma SRL
11 Ion Mihalache Blvd
Bucharest 1, Romania 011171Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Rev. A 9/2022
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUDARABINE PHOSPHATE
fludarabine phosphate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45963-609 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUDARABINE PHOSPHATE (UNII: 1X9VK9O1SC) (FLUDARABINE - UNII:P2K93U8740) FLUDARABINE PHOSPHATE 50 mg in 2 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45963-609-55 1 in 1 CARTON 01/05/2015 1 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078610 01/05/2015 Labeler - Actavis Pharma, Inc. (119723554)


