Label: acetaminophen, caffeine and dihydrocodeine bitartrate- Acetaminophen, Caffeine and Dihydrocodeine Bitartrate tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 0525-0032-01, 0525-0032-05 - Packager: MIKART, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
Drug Label Information
Updated October 6, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets are supplied in tablet form for oral administration.
Each tablet contains:
Acetaminophen..........................................................712.8 mg
Caffeine.....................................................................60 mg
Dihydrocodeine Bitartrate*..........................................32 mg
(*Warning: May be habit forming)
Acetaminophen (4'-hydroxyacetanilide), a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula:
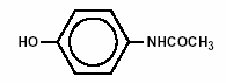
C8H9NO2 M.W. = 151.17
Caffeine (1,3,7-trimethylxanthine), a bitter, white powder or white-glistening needles, is a central nervous system stimulant. It has the following structural formula:
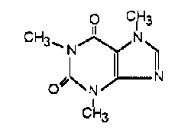
C8H10N4O2 (anhydrous) M.W. = 194.19
Dihydrocodeine bitartrate (4,5α-Epoxy-3-methoxy-17-methylmorphinan-6α-ol (+)-tartrate), an odorless, fine white powder is an opioid analgesic. It has the following structural formula:
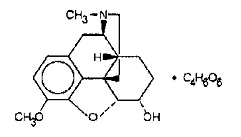
C18H23NO3•C4H6O6 M.W. = 451.48
In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, stearic acid, D&C Red #27 Aluminum Lake, D&C Red #30 Aluminum Lake, and FD&C Blue #1 Aluminum Lake.
-
CLINICAL PHARMACOLOGY
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets contain dihydrocodeine which is a semi-synthetic narcotic analgesic related to codeine, with multiple actions qualitatively similar to those of codeine; the most prominent of these involve the central nervous system and organs with smooth muscle components. The principal action of therapeutic value is analgesia.
This combination product also contains acetaminophen, a non-opiate, non-salicylate analgesic, and antipyretic.
This combination product contains caffeine as an analgesic adjuvant. Caffeine is also a central nervous system and cardiovascular stimulant.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
This combination product is contraindicated in patients with hypersensitivity to dihydrocodeine, codeine, acetaminophen, caffeine, or any of the inactive components listed above, or in any situation where opioids are contraindicated including significant respiratory depression (in unmonitored settings or in the absence of resuscitative equipment), acute or severe bronchial asthma or hypercapnia, and paralytic ileus.
-
WARNINGS
Usage in Ambulatory Patients
Dihydrocodeine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
Respiratory Depression
Respiratory depression is the most dangerous acute reaction produced by opioid agonist preparations, although it is rarely severe with usual doses. Opioids decrease the respiratory rate, tidal volume, minute ventilation, and sensitivity to carbon dioxide. Respiratory depression occurs most frequently in elderly or debilitated patients, usually after large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration. This combination product should be used with caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale and in patients with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or respiratory depression. In such patients alternative non-opioid analgesics should be considered, and opioids should be administered only under careful medical supervision at the lowest effective dose.
Head Injury
This combination product should be used cautiously in the presence of head injury or increased intracranial pressure. The effects of opioids on pupillary response and consciousness may obscure neurologic signs of increases in intracranial pressure in patients with head injuries. The respiratory depressant effects including carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, intracranial lesions, or other causes of increased intracranial pressure.
Hypotensive Effect
Dihydrocodeine, like all opioid analgesics, may cause hypotension in patients whose ability to maintain blood pressure has been compromised by a depleted blood volume or who receive concurrent therapy with drugs such as phenothiazines or other agents which compromise vasomotor tone. Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets may produce orthostatic hypotension in ambulatory patients. This combination product should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Drug Dependence
Dihydrocodeine can produce drug dependence of the codeine type and has the potential of being abused (see DRUG ABUSE AND DEPENDENCE).
-
PRECAUTIONS
General
Selection of patients for treatment with Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets should be governed by the same principles that apply to the use of similar opioid/non-opioid fixed combination analgesics. As with any such opioid analgesic, the dosing regimen should be adjusted for each patient (see DOSAGE AND ADMINISTRATION). This combination product should be used with caution in elderly or debilitated patients or those with any of the following conditions: acute alcoholism; adrenocortical insufficiency (e.g., Addison’s disease); asthma; central nervous system depression or coma; chronic obstructive pulmonary disease; decreased respiratory reserve (including emphysema, severe obesity, cor pulmonale, or kyphoscoliosis); delirium tremens; head injury; hypotension; increased intracranial pressure; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; and toxic psychosis. The benefits and risks of using opioids in patients taking monoamine oxidase inhibitors and in those with a history of drug abuse should be carefully considered. The administration of an analgesic containing an opioid may obscure the diagnosis or clinical course in patients with acute abdominal conditions. This combination product may aggravate convulsions in patients with convulsive disorders and, like all opioids, may induce or aggravate seizures in some clinical settings.
Acetaminophen is relatively non-toxic at therapeutic doses, but should be used with caution in patients with severe renal or hepatic disease.
Care should be observed when using large doses of acetaminophen in malnourished patients or those with a history of chronic alcohol abuse because they may be more susceptible to hepatic damage similar to that observed with toxic overdosage.
Caffeine in high doses may produce central nervous system and cardiovascular stimulation and gastrointestinal irritation.
Drug-Drug Interactions
Dihydrocodeine with Other Central Nervous System Depressants
Patients receiving other opioid analgesics, sedatives or hypnotics, muscle relaxants, general anesthetics, centrally acting anti-emetics, phenothiazines or other tranquilizers, or alcohol concomitantly with this combination product may exhibit additive depressant effects on the central nervous system. When such combined therapy is contemplated, the dosage of one or both agents should be reduced.
Dihydrocodeine with Monoamine Oxidase Inhibitors
Dihydrocodeine, like all opioid analgesics, interacts with monoamine oxidase inhibitors causing central nervous system excitation and hypertension.
Dihydrocodeine with Mixed Agonist/Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol and buprenorphine) may reduce the analgesic effect of this combination product.
Acetaminophen-Drug Interactions
Chronic and excessive consumption of alcohol may increase the hepatotoxic risk of acetaminophen. The potential for hepatotoxicity with acetaminophen also may be increased in patients receiving anticonvulsants that induce hepatic microsomal enzymes (including phenytoin, barbiturates, and carbamazepine) or isoniazid. Chronic ingestion of large doses of acetaminophen may slightly potentiate the effects of warfarin- and indandione-derivative anticoagulants. Severe hypothermia is possible in patients receiving acetaminophen concomitantly with phenothiazines.
Caffeine-Drug Interactions
Caffeine may enhance the cardiac inotropic effects of beta-adrenergic stimulating agents. Coadministration of caffeine and disulfiram may lead to a substantial decrease in caffeine clearance. Caffeine may increase the metabolism of other drugs such as phenobarbital and aspirin. Caffeine accumulation may occur when products or foods containing caffeine are consumed concomitantly with quinolones such as ciprofloxacin.
Information for Patients/Caregivers
Patients receiving Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets should be given the following information:
- Patients should be advised that Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets may impair the mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Patients should be advised to report adverse experiences occurring during therapy.
- Patients should be advised not to adjust the dose of Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets without consulting the prescribing professional.
- Patients should not combine Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets with alcohol or other central nervous system depressants (sleep aids, tranquilizers) except by the orders of the prescribing physician, because additive effects may occur.
- Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
- Patients should be advised that Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets are a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
Pregnancy
Teratogenic Effects - Pregnancy Category C.
Animal reproduction studies have not been conducted with Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets. It is also not known whether this combination product can cause fetal harm when administered to pregnant women or can affect reproduction capacity in males and females. This combination product should be given to a pregnant woman only if clearly needed, especially during the first trimester.
Labor and Delivery
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets are not recommended for use by women during and immediately before labor and delivery because oral opioids may cause respiratory depression in the newborn.
Nursing Mothers
Dihydrocodeine bitartrate, acetaminophen and caffeine are excreted in breast milk in small amounts, but the significance of their effects on nursing infants is not known. Because of the potential for serious adverse reactions in nursing infants from this combination product, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets in pediatric patients have not been established.
Geriatric Use
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets should be given with caution to the elderly.
Hepatic Impairment
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets should be given with caution to patients with hepatic insufficiency. Since dihydrocodeine is metabolized by the liver and since acetaminophen potentially causes hepatotoxicity, the effects of this combination product should be monitored closely in such patients.
-
ADVERSE REACTIONS
Dihyrocodeine
The most frequently observed reactions include lightheadedness, dizziness, drowsiness, headache, fatigue, sedation, sweating, nausea, vomiting, constipation, pruritus, and skin reactions. With the exception of constipation, tolerance develops to most of these effects. Other reactions that have been observed with dihydrocodeine or other opioids include respiratory depression, orthostatic hypotension, cough suppression, confusion, diarrhea, miosis, abdominal pain, dry mouth, indigestion, anorexia, spasm of biliary tract, and urinary retention. Physical and psychological dependence are possibilities. Hypersensitivity reactions (including anaphylactoid reactions), hallucinations, vivid dreams, granulomatous interstitial nephritis, severe narcosis and acute renal failure have been reported rarely during dihydrocodeine administration.
Acetaminophen
Acetaminophen in therapeutic doses rarely causes adverse reactions. The most serious adverse reaction is hepatotoxicity from overdosage (see OVERDOSAGE). Thrombocytopenia, leukopenia, pancytopenia, neutropenia, thrombocytopenic purpura, and agranulocytosis have been reported in patients receiving acetaminophen or ρ-aminophenol derivatives. Hypersensitivity reactions including urticarial or erythematous skin reactions, laryngeal edema, angioedema, or anaphylactoid reactions are rare.
Caffeine
Adverse reactions associated with caffeine use include anxiety, anxiety neurosis, excitement, headaches, insomnia, irritability, lightheadedness, restlessness, tenseness, tremor, extrasystoles, palpitations, tachycardia, diarrhea, nausea, stomach pain, vomiting, diuresis, urticaria, scintillating scotoma, and tinnitus.
-
DRUG ABUSE AND DEPENDENCE
This combination product is subject to the provisions of the Controlled Substance Act, and has been placed in Schedule III.
Dihydrocodeine can produce drug dependence of the codeine type and therefore has the potential of being abused. Like other opioid analgesics, dihydrocodeine may produce subjective effects other than analgesia (e.g., euphoria, relaxation), which may contribute to abuse by some patients. Psychological dependence, physical dependence, and tolerance may develop upon repeated administration of dihydrocodeine, and it should be prescribed and administered with the same degree of caution appropriate to the use of other oral opioid analgesic medications. Symptoms of dihydrocodeine withdrawal consist of irritability, restlessness, insomnia, diaphoresis, anxiety and palpitations.
Prolonged, high intake of caffeine may produce tolerance and habituation. Physical signs of withdrawal, such as headaches, irritation, nervousness, anxiety, and dizziness may occur upon abrupt discontinuation.
-
OVERDOSAGE
Following an acute overdosage with Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets, toxicity may result from the dihydrocodeine, acetaminophen, or, less likely, caffeine component. An overdose is a potentially lethal polydrug overdose situation, and consultation with a regional poison control center is recommended. A listing of the poison control centers can be found in standard references such as the Physician’s Desk Reference®.
Signs and Symptoms and Laboratory Findings
Toxicity from dihydrocodeine is typical of opioids and includes pinpoint pupils, respiratory depression, and loss of consciousness. Convulsions, cardiovascular collapse, and death may occur. A single case of acute rhabdomyolysis associated with an overdose of dihydrocodeine has been reported. With acetaminophen, dose-dependent potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may occur. Early symptoms of hepatotoxicity include nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours after ingestion. Acute caffeine poisoning may cause insomnia, restlessness, tremor, delirium, tachycardia, extrasystoles, and seizures.
Because overdose information on this combination product is limited, it is unclear which of the signs and symptoms of toxicity would manifest in any particular overdose situation.
Treatment
Immediate treatment of an overdosage of Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced with syrup of ipecac, if the patient is alert and has adequate laryngeal reflexes. Oral activated charcoal should follow. The first dose of charcoal should be accompanied by an appropriate cathartic. Gastric lavage may be necessary. Hypotension is usually hypovolemic and should be treated with fluids. Endotracheal intubation and artificial respiration may be necessary. Peritoneal or hemodialysis may be necessary. If hypoprothrombinemia occurs, Vitamin K should be administered.
A pure opioid antagonist, such as naloxone or nalmefene, is a specific antidote against respiratory depression which results from opioid overdose. Opioid antagonists should not be given in the absence of clinically significant respiratory or circulatory depression secondary to opioid overdose. They should be administered cautiously to persons who are known, or suspected to be, physically dependent on any opioid agonist including Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. The prescribing information for the specific opioid antagonist should be consulted for details of their proper use.
In adults and adolescents, regardless of the quantity of acetaminophen reported to have been ingested, acetylcysteine should be administered immediately if 24 hours or less have elapsed from the reported time of ingestion. It is not advisable to await the plasma concentration determination of acetaminophen before administering acetylcysteine. Serum liver enzyme levels should be measured. Therapy in children involves a similar treatment scheme; however, a regional Poison Control Center should be contacted.
No specific antidote is available for caffeine. In addition to the supportive measures above, administration of demulcents such as aluminum hydroxide gel may diminish gastrointestinal irritation. Seizures may be treated with intravenous diazepam or a barbiturate.
-
DOSAGE AND ADMINISTRATION
The usual adult dosage is one (1) Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablet orally every four (4) hours, as needed. Dosage should be adjusted according to the severity of the pain and the response of the patient. No more than one tablet should be taken in a four hour period. No more than five (5) doses, or five (5) tablets should be taken in a 24-hour period.
-
HOW SUPPLIED
Acetaminophen, Caffeine and Dihydrocodeine Bitartrate Tablets, containing acetaminophen 712.8 mg, caffeine 60 mg and dihydrocodeine bitartrate* 32 mg, (*Warning: May be habit-forming), are supplied in bottles of 100 tablets NDC 0525-0032-01, and in bottles 500 tablets, NDC 0525-0032-05. Tablets are lavender, oval-shaped, single-scored and are debossed “PAL” on one side and “032” on the other side.
Store at controlled room temperature, 15°-30°C (59°-86°F). Protect from moisture.
Dispense in a tight, light-resistant container with a child resistant closure.
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN, CAFFEINE AND DIHYDROCODEINE BITARTRATE
acetaminophen, caffeine and dihydrocodeine bitartrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0525-0032 Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) 712.8 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) 60 mg DIHYDROCODEINE BITARTRATE (UNII: 8LXS95BSA9) (CODEINE - UNII:Q830PW7520) 32 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE () CROSPOVIDONE () D & C RED # 30 ALUMINUM LAKE () D & C RED #27 ALUMINUM LAKE () FD & C BLUE # 1 ALUMINUM LAKE () MAGNESIUM STEARATE (POWDER) () MICROCRYSTALLINE CELLULOSE () POVIDONE () PREGELATINIZED STARCH () STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color PURPLE (LAVENDER) Score 2 pieces Shape OVAL (OVAL) Size 19mm Flavor Imprint Code PAL;032 Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0525-0032-05 500 in 1 BOTTLE, PLASTIC 2 NDC:0525-0032-01 100 in 1 BOTTLE, PLASTIC Labeler - MIKART, INC.
