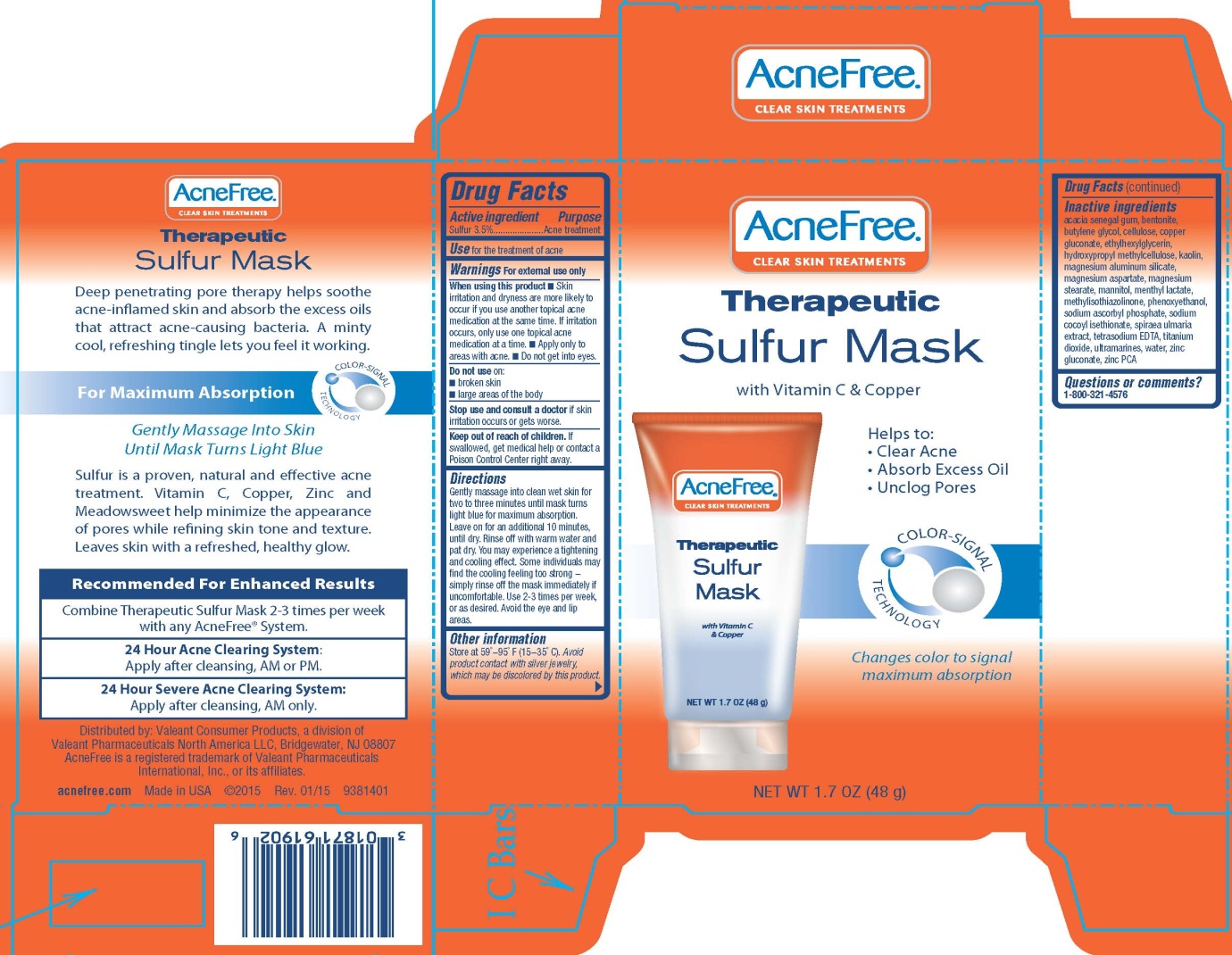
ACNEFREE THERAPEUTIC SULFUR MASK- sulfur cream
Bausch Health US, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
When using this product
- •
- Skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- •
- Apply only to areas with acne.
- •
- Do not get into eyes.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Gently massage into clean wet skin for two to three minutes until mask turns light blue for maximum absorption. Leave on for an additional 10 minutes, until dry. Rinse off with warm water and pat dry. You may experience a tightening and cooling effect. Some individuals may find the cooling feeling too strong - simply rinse off the mask immediately if uncomfortable. Use 2-3 times per week, or as desired. Avoid the eye and lip areas.
Other information
Store at 59° - 95° F (15-35° C).
Avoid product contact with silver jewelry, which may be discolored by this product.
Inactive ingredients
acacia senegal gum, bentonite, butylene glycol, cellulose, copper gluconate, ethylhexylglycerin, hydroxypropyl methylcellulose, kaolin, magnesium aluminum silicate, magnesium aspartate, magnesium stearate, mannitol, menthyl lactate, methylisothiazolinone, phenoxyethanol, sodium ascorbyl phosphate, sodium cocoyl isethionate, spiraea ulmaria extract, tetrasodium EDTA, titanium dioxide, ultramarines, water, zinc gluconate, zinc PCA
Questions or Comments?
1-800-321-4576
Distributed by: Valeant Consumer Products, a division of
Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807
AcneFree is a registered trademark of Valeant Pharmaceuticals
International, Inc., or its affiliates.
acnefree.com
Made in USA
©2015
Rev. 01/15
9381401
| ACNEFREE THERAPEUTIC SULFUR MASK
sulfur cream |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bausch Health US, LLC (831922468) |