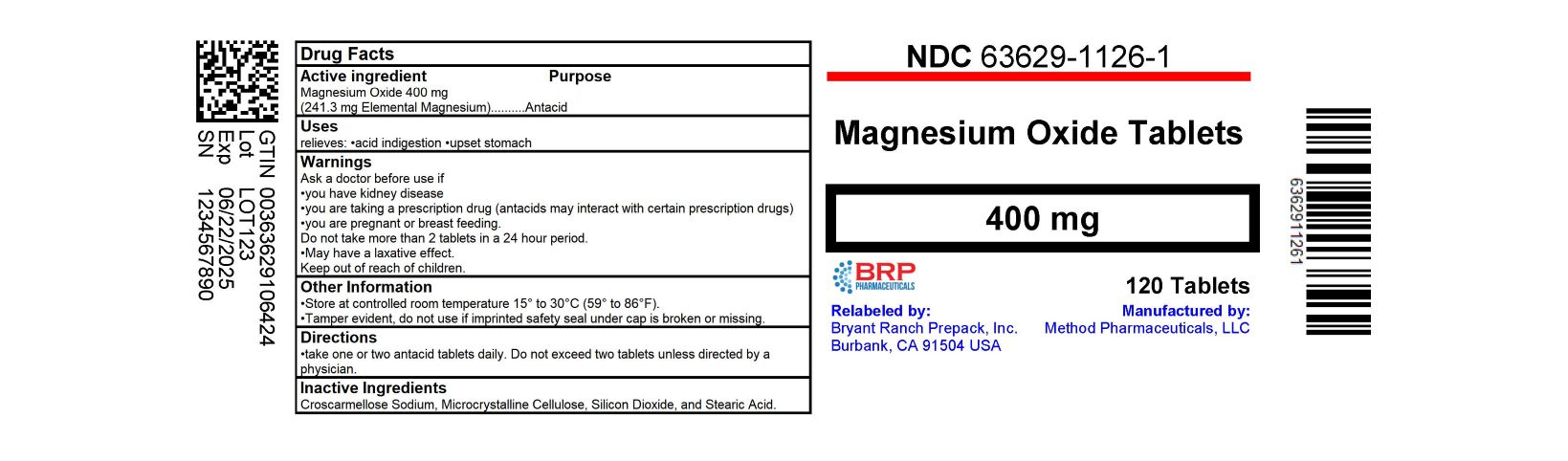
Label: MAGNESIUM OXIDE tablet
- NDC Code(s): 63629-1126-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 58657-120
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAGNESIUM OXIDE
magnesium oxide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63629-1126(NDC:58657-120) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 400 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code 120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-1126-1 120 in 1 BOTTLE; Type 0: Not a Combination Product 10/05/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 07/25/2018 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-1126) , RELABEL(63629-1126)