AUROMUCUS - FAST MAXIMUM SEVERE CONGESTION AND COUGH - dextromethorphan hydrobromide, guaifenesin and phenylephrine hydrochloride solution
Aurohealth LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
AuroMucus - Fast Maximum Severe Congestion and Cough
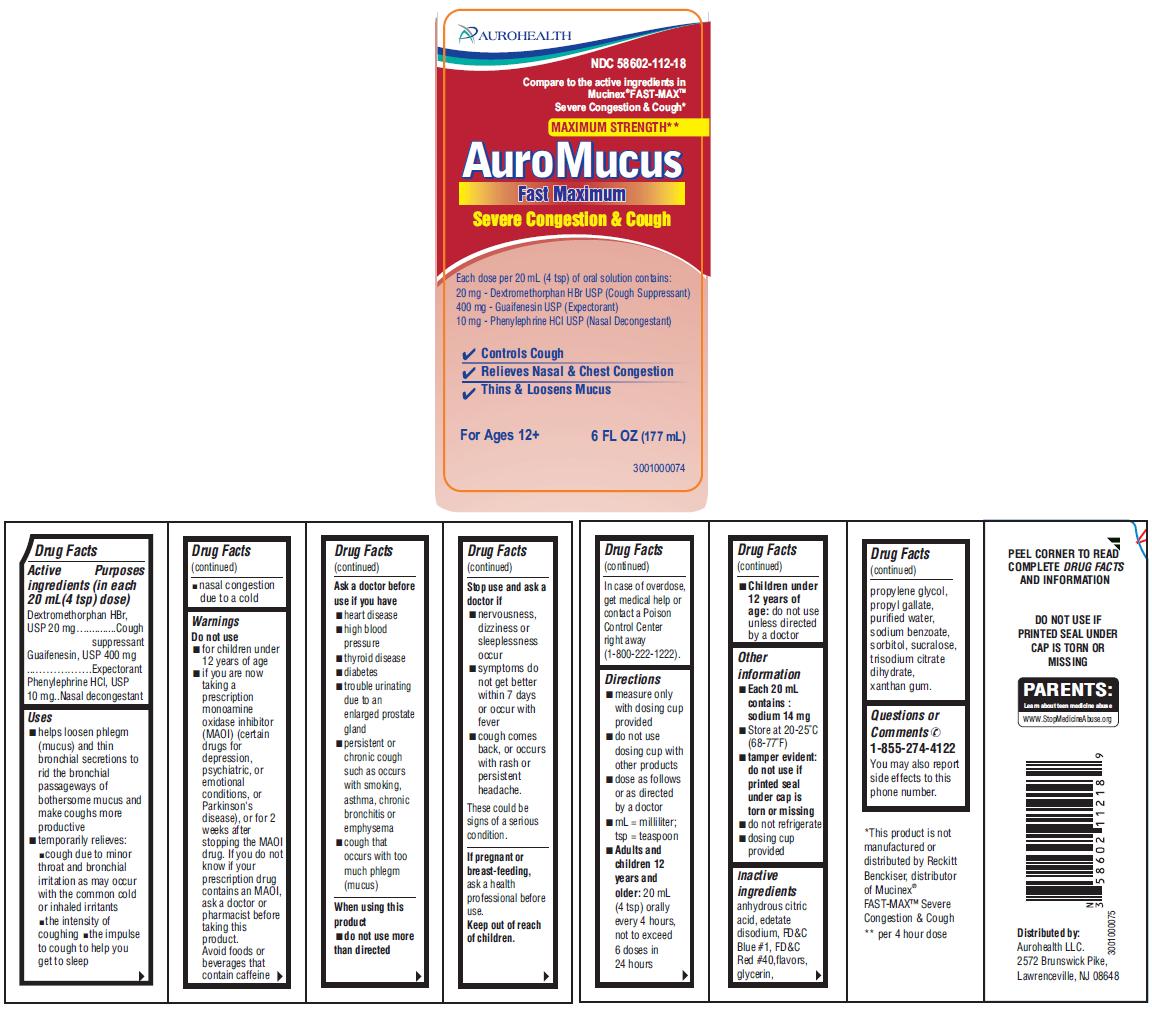
Drug Facts
Active ingredients (in each 20 mL (4 tsp) dose)
Dextromethorphan HBr, USP 20 mg
Guaifenesin, USP 400 mg
Phenylephrine HCl, USP 10 mg
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
- nasal congestion due to a cold
Warnings
Do not use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. Avoid foods or beverages that contain caffeine
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- nervousness, dizziness or sleeplessness occur
- symptoms do not get better within 7 days or occur with fever
- cough comes back, or occurs with rash or persistent headache.
These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
- mL = milliliter; tsp = teaspoon
- Adults and children 12 years and older: 20 mL (4 tsp) orally every 4 hours, not to exceed 6 doses in 24 hours
- Children under 12 years of age: do not use unless directed by a doctor
Other information
- Each 20 mL contains: sodium 14 mg
- Store at 20-25°C (68-77°F)
- tamper evident: do not use if printed seal under cap is torn or missing
- do not refrigerate
- dosing cup provided
Inactive ingredients
anhydrous citric acid, edetate disodium, FD&C Blue #1, FD&C Red #40, flavors, glycerin, propylene glycol, propyl gallate, purified water, sodium benzoate, sorbitol, sucralose, trisodium citrate dihydrate, xanthan gum
Questions or Comments
1-855-274-4122
You may also report side effects to this phone number.
Distributed by:
Aurohealth LLC.
2572 Brunswick Pike,
Lawrenceville, NJ 08648
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 6 FL OZ (177 mL Bottle)
AUROHEALTH
NDC 58602-112-18
Compare to the active ingredients in
Mucinex® FAST-MAXTM
Severe Congestion & Cough*
MAXIMUM STRENGTH**
AuroMucus
Fast Maximum
Severe Congestion & Cough
Each dose per 20 mL (4 tsp) of oral solution contains:
20 mg - Dextromethorphan HBr USP (Cough Suppressant)
400 mg - Guaifenesin USP (Expectorant)
10 mg - Phenylephrine HCl USP (Nasal Decongestant)
- Controls Cough
- Relieves Nasal & Chest Congestion
- Thins & Loosens Mucus
For Ages 12+ 6 FL OZ (177 mL)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 9 FL OZ (266 mL Bottle)
AUROHEALTH
NDC 58602-112-21
Compare to the active ingredients in
Mucinex® FAST-MAXTM
Severe Congestion & Cough*
MAXIMUM STRENGTH**
AuroMucus
Fast Maximum
Severe Congestion & Cough
Each dose per 20 mL (4 tsp) of oral solution contains:
20 mg – Dextromethorphan HBr USP (Cough Suppressant)
400 mg - Guaifenesin USP (Expectorant)
10 mg - Phenylephrine HCl USP (Nasal Decongestant)
- Controls Cough
- Relieves Nasal & Chest Congestion
- Thins & Loosens Mucus
For Ages 12+ 9 FL OZ (266 mL)

| AUROMUCUS - FAST MAXIMUM SEVERE CONGESTION AND COUGH
dextromethorphan hydrobromide, guaifenesin and phenylephrine hydrochloride solution |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurohealth LLC | 078728447 | MANUFACTURE(58602-112) | |