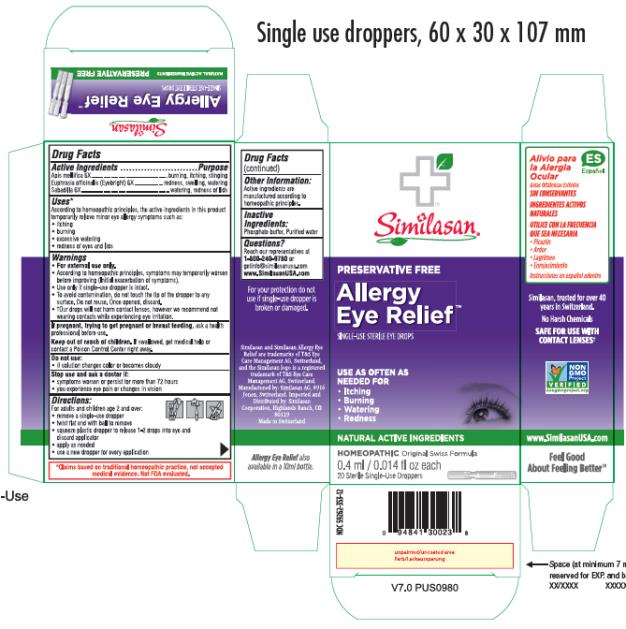
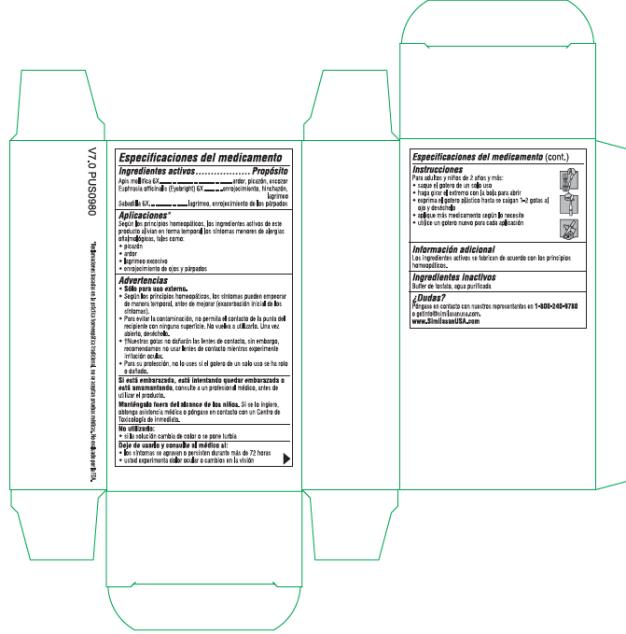
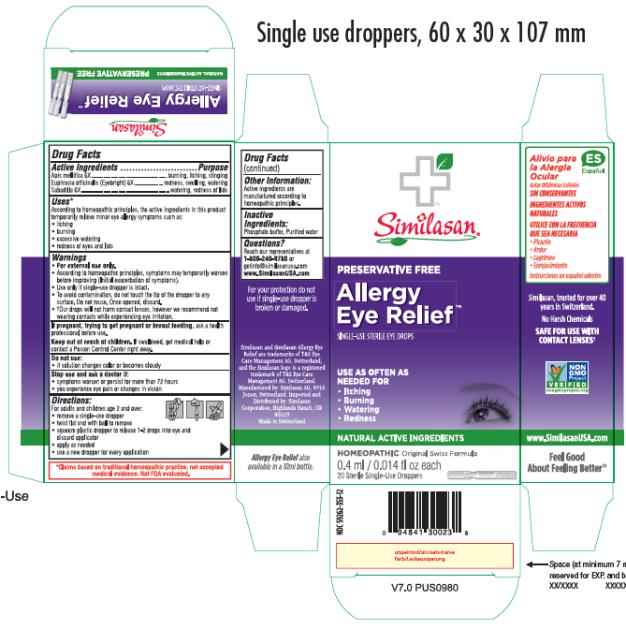
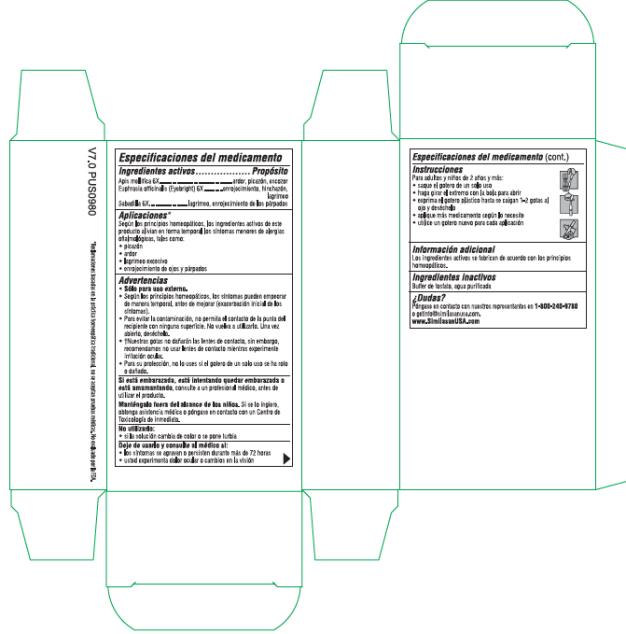
Label: ALLERGY EYE RELIEF- apis mellifera and euphrasia stricta and schoenocaulon officinale seed solution/ drops
- NDC Code(s): 59262-353-12
- Packager: Similasan Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 28, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses*
-
Warnings
• For external use only.
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
• Use only if single-use dropper is intact.
• To avoid contamination, do not touch the tip of the dropper to any surface. Do not reuse. Once opened, discard.
• †Our drops will not harm contact lenses, however we recommend not wearing contacts while experiencing eye irritation.
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY EYE RELIEF
apis mellifera and euphrasia stricta and schoenocaulon officinale seed solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-353 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 0.4 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 0.4 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 6 [hp_X] in 0.4 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-353-12 20 in 1 BOX 07/02/2013 1 0.4 mL in 1 VIAL, SINGLE-USE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/02/2013 Labeler - Similasan Corporation (111566530)