ARTISS- fibrinogen human thrombin human
Baxalta US Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARTISS safely and effectively. See full prescribing information for ARTISS
ARTISS [Fibrin Sealant (Human)] For Topical Use Only Frozen solution and lyophilized powder for solution for topical application includes DUPLOJECT Initial U.S. Approval: 2008 RECENT MAJOR CHANGESINDICATIONS AND USAGE
DOSAGE AND ADMINISTRATIONFor Topical Use Only. Do Not Inject (2). Apply on surface of prepared wound beds only (2.3) ARTISS Kit (Freeze-Dried) requires reconstitution prior to use (2.1) ARTISS Pre-filled Syringe (Frozen) requires thawing prior to use (2.2) Apply as a thin layer using the Easyspray and Spray Set (2.3, 5.2) Dosage: 2 mL will cover approximately 100 cm2 surface area (2) Vials and pre-filled syringes are for single use only. Discard unused contents (2.3) DOSAGE FORMS AND STRENGTHSARTISS is available as a two-component fibrin sealant, including Sealer Protein (Human) and Thrombin (Human), in two dosage forms, 2 mL, 4 mL and 10 mL Freeze-Dried Kit and 2 mL, 4 mL and 10 mL Frozen Solution in Pre-filled Syringe (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSAdverse reactions reported during clinical trials in greater than 1% for subjects were: Burns: skin graft failure, hematoma and pruritus (6.1) Facial Rhytidectomy: hematoma/seroma (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare Corporation at 1-866-888-2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSPregnancy: No human or animal data. Use ARTISS only if clearly needed (8.1) See 17 for PATIENT COUNSELING INFORMATION. Revised: 8/2011 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
FOR TOPICAL USE ONLY – DO NOT INJECT.
The amount of ARTISS to be applied must be individualized by the treating physician based on the size of the surface to be covered. The approximate surface areas covered by each package size of ARTISS are:
| Approximate area requiring skin graft fixation | Required package size of ARTISS |
| 100 cm2 | 2 mL |
| 200 cm2 | 4 mL |
| 500 cm2 | 10 mL |
It is recommended that every time a patient receives a dose of ARTISS the name and lot number (batch number) of the product are documented in order to maintain link between the patient and product batch.
2.1 Preparation of ARTISS Kit (Freeze-Dried)
During preparation of ARTISS Kit:
DO NOT EXPOSE TO TEMPERATURES ABOVE 37°C
DO NOT REFRIGERATE OR FREEZE AFTER RECONSTITUTION
Do not use iodine or heavy metal containing preparations such as betadine for disinfection of vial stoppers. Allow alcohol-based disinfectants to evaporate before puncturing stopper.
After reconstitution, the product must be used within 4 hours.
Use separate syringes for reconstituting Sealer Protein and Thrombin solutions and for application to prevent premature clotting.
ARTISS Kit contains the following substances in four separate vials:
- -Sealer Protein Concentrate (Human)
- -Fibrinolysis Inhibitor Solution (Synthetic)
- -Thrombin (Human)
- -Calcium Chloride Solution
Freeze-dried Sealer Protein Concentrate and Thrombin are reconstituted in Fibrinolysis Inhibitor Solution and Calcium Chloride Solution, respectively. The Sealer Protein Solution and Thrombin Solution are then combined using the DUPLOJECT Preparation and Application System, or an equivalent delivery device cleared by FDA for use with ARTISS to form the Fibrin Sealant.
Prewarming ARTISS Kit with FIBRINOTHERM
If a FIBRINOTHERM device is not available, contact Baxter (1-800-423-2090) for assistance. See FIBRINOTHERM manual for complete operating instructions.
- Plug the FIBRINOTHERM Heating and Stirring Device into an electrical socket and activate the warmer (amber switch). Ensure that the stirring mechanism of the FIBRINOTHERM device is initially switched off (green switch).
- Place all four vials from the ARTISS Kit into the prewarmed wells of the FIBRINOTHERM, using the appropriately sized adapter rings, and allow the vials to warm for up to 5 minutes (room temperature product may take less time).
Preparation of Sealer Protein Solution with FIBRINOTHERM
- Remove the flip-off caps from the vial containing the Sealer Protein Concentrate and the vial containing the Fibrinolysis Inhibitor Solution, disinfect the rubber stoppers of both vials with a germicidal solution and allow to dry.
- Transfer the Fibrinolysis Inhibitor Solution into the vial containing the freeze-dried Sealer Protein Concentrate using the sterile reconstitution components provided with the DUPLOJECT Preparation and Application System, or an equivalent device cleared by FDA for use with ARTISS (see directions provided with the device system for specific reconstitution instructions). Gently swirl the vial to ensure that the freeze-dried material is completely soaked.
- Place the vial into the largest opening of the FIBRINOTHERM device with the appropriate adaptor. Switch on the stirrer (green switch) and allow the vial contents to stir until all Sealer Protein Concentrate is dissolved.
- Reconstitution of the freeze-dried Sealer Protein Concentrate is complete as soon as no undissolved particles are visible. Otherwise, return the vial to the FIBRINOTHERM device and agitate for a few more minutes until the solution appears homogeneous.
Notes:
- Do not use the Sealer Protein Concentrate until it has fully dissolved. If the Sealer Protein Concentrate has not dissolved within 20 minutes using the FIBRINOTHERM device, discard the vial and prepare a fresh kit. Excessive stirring (20 minutes or more) may compromise product quality.
- If not used promptly, keep the Sealer Protein Solution at 37°C without stirring. To ensure homogeneity, switch on the stirrer of the FIBRINOTHERM device shortly before drawing up the solution.
Preparation of Thrombin Solution with FIBRINOTHERM
- Remove the flip-off caps from the vial containing Thrombin and the vial containing Calcium Chloride Solution, disinfect the rubber stoppers of both vials with a germicidal solution and allow to dry.
- Transfer the contents of the vial with Calcium Chloride Solution into the vial containing the freeze-dried Thrombin using the sterile reconstitution components provided with the DUPLOJECT Preparation and Application System, or an equivalent device cleared by FDA for use with ARTISS (see directions provided with the device system for specific reconstitution instructions).
- Swirl briefly.
- Place the vial into the adapted opening of the FIBRINOTHERM device.
- Reconstitution of Thrombin is complete when all of the Thrombin concentrate is dissolved.
- Keep the Thrombin Solution at 37°C until used.
Transferring to the Sterile Field
For transfer of the Sealer Protein Solution and the Thrombin Solution to the sterile field, the scrub nurse should withdraw the solutions while the circulating nurse holds the non-sterile vials. The solutions should be withdrawn slowly by firm constant aspiration to reduce the risk of large air bubbles.
2.2 Preparation of ARTISS Pre-filled Syringe (Frozen)
During preparation of ARTISS (frozen):
DO NOT EXPOSE TO TEMPERATURES ABOVE 37°C
DO NOT MICROWAVE
DO NOT REFRIGERATE OR RE-FREEZE AFTER THAWING
Do not use ARTISS (frozen) unless it is completely thawed and warmed (liquid consistency).
Do not remove the protective syringe cap until thawing is complete and the application tip is ready to be attached.
ARTISS (frozen) can be prepared (thawed) using one of two options:
Room Temperature Thawing
Approximate thawing times when using this method are:
| Pack Size | Room Temperature (In Pouches) |
| 2 mL | 60 minutes |
| 4 mL | 110 minutes |
| 10 mL | 160 minutes |
Unopened pouches, thawed at room temperature, may be stored for up to 14 days at 15-25°C.
Prior to use, the product should be warmed to 33-37°C:
| Pack Size | 33°C to 37°C Incubator (In Pouches) |
| 2 mL | 15 minutes |
| 4 mL | 25 minutes |
| 10 mL | 35 minutes |
Quick Thawing
Thawing on the sterile field using a water bath
33°C to 37°C sterile water bath - transfer the inner pouch to the sterile field, remove pre-filled syringe from inner pouch and place directly into sterile water bath. Ensure the contents of the pre-filled syringe are completely immersed under the water.
Approximate thawing times when using this method are:
| Pack Size | 33°C to 37°C Sterile Water Bath (Pouches Removed) |
| 2 mL | 5 minutes |
| 4 mL | 5 minutes |
| 10 mL | 12 minutes |
Thawing off the sterile field using a water bath
33°C to 37°C non-sterile water bath in two pouches - maintain the pre-filled syringe in both pouches and place into a water bath off the sterile field for appropriate time. Ensure the pouches remain submerged throughout thawing. Remove from the water bath after thawing, dry external pouch and transfer inner pouch with pre-filled syringe onto the sterile field.
Approximate thawing times when using this method are:
| Pack Size | 33°C to 37°C Non-Sterile Water Bath (In Pouches) |
| 2 mL | 30 minutes |
| 4 mL | 40 minutes |
| 10 mL | 80 minutes |
Thawing off the sterile field using an incubator
33°C to 37°C incubator in pouches – maintain the pre-filled syringe in both pouches and place into an incubator for appropriate time. Remove from incubator after thawing and transfer inner pouch with pre-filled syringe onto the sterile field.
Approximate thawing times when using this method are:
| Pack Size | 33°C to 37°C Incubator (In Pouches) |
| 2 mL | 40 minutes |
| 4 mL | 85 minutes |
| 10 mL | 105 minutes |
Maintain the product at 33-37°C until use. If product is removed from original pouch or warmed to 33-37°C it must be used within 12 hours.
2.3 Method of Application
Apply ARTISS using the Easyspray and Spray Set, or an equivalent device cleared by FDA for application of ARTISS. See additional instructions for use provided with the spray set.
The wound surface should be as dry as possible before application.
Ensure that parts of the body outside the desired application area are sufficiently covered to prevent tissue adherence at undesired site.
Apply ARTISS as a thin layer to avoid the formation of excess granulation tissue and to ensure gradual absorption of the polymerized fibrin sealant.
The aerosolized sealant should be applied to the wound in a painting motion from side to side to achieve a single thin application. The wound bed will glisten in the area to which fibrin sealant has been applied.
Any areas not covered by fibrin sealant will be clearly visible.
The skin flap or graft should be attached to the wound bed immediately after ARTISS has been sprayed. The surgeon has up to 60 seconds to manipulate and position the flap or graft prior to polymerization. The initial amount of the product to be applied should be sufficient to cover the intended application area.
The application can be repeated, if necessary, to any small areas that may not have been previously treated. To prevent adherence, wet gloves with normal saline before product contact.
After the flap or graft has been positioned, hold in the desired position by gentle compression for at least 3 minutes to ensure ARTISS sets properly and adheres firmly to the surrounding tissue. The solidified fibrin sealant reaches its final strength in approximately 2 hours after application.
The cannulas included with the DUPLOJECT Preparation and Application System or DUO Set may be used for small wounds or for edges of a skin graft that did not adhere to the wound bed (see WARNINGS/PRECAUTIONS Application Precautions (5.3)). Immediately before application, expel and discard the first several drops from the application cannula to ensure adequate mixing of the Sealer Protein and Thrombin solutions in cases where very small volumes (1-2 drops) are administered.
- Freeze-Dried: Refer to instructions for use provided with the DUPLOJECT Preparation and Application System.
- Frozen: DUO Set Instructions (see Figure 1 below):
- Insert plunger into syringe barrel.
- Firmly connect the two syringe nozzles to the joining piece and secure it by fastening the tether strap to the syringe.
- Fit an application cannula to the joining piece.
If application of ARTISS is interrupted, replace the cannula immediately before application is resumed.
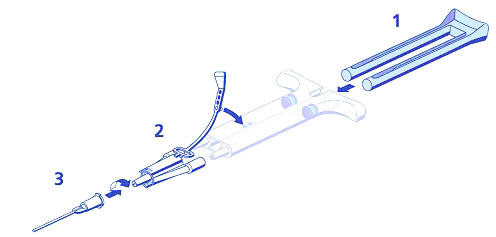
Figure 1 DUO SET A
Vials and pre-filled syringes are for single use only. Discard unused contents.
3 DOSAGE FORMS AND STRENGTHS
ARTISS is available as a two-component fibrin sealant, including Sealer Protein (Human) and Thrombin (Human), in two dosage forms 2 mL, 4 mL and 10 mL Freeze-Dried Kit and 2 mL, 4 mL and 10 mL Frozen Solution in Pre-filled Syringe.
The reconstituted solution or pre-filled syringe contains:
Sealer Protein Solution
| Total protein: | 96 – 125 mg/mL | |
| Fibrinogen: | 67 – 106 mg/mL | |
| Fibrinolysis Inhibitor (Synthetic): | 2250 – 3750 KIU/mL | |
| Other ingredients include: human albumin, tri-sodium citrate, histidine, niacinamide, polysorbate 80 and water for injection (WFI). | ||
Thrombin Solution
|
||
| Thrombin (Human): | 2.5 – 6.5 units/mL* | |
| Calcium Chloride: | 36 – 44 µmol/mL | |
| Other ingredients include: human albumin, sodium chloride and water for injection (WFI). | ||
4 CONTRAINDICATIONS
4.1 Intravascular Application
Do not inject ARTISS directly into blood vessels. Intravascular application of ARTISS may result in life-threatening thromboembolic events.
4.2 Aprotinin Hypersensitivity
Do not use ARTISS in individuals with a known hypersensitivity to aprotinin and/or hypersensitivity to any of the active substances or excipients (see WARNINGS/PRECAUTIONS, Hypersensitivity/Allergic/Anaphylactic Reactions (5.1) and ADVERSE REACTIONS (6)).
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity/Allergic/Anaphylactic Reactions
Hypersensitivity or allergic/anaphylactoid reactions may occur with the use of ARTISS. Cases have been reported in post marketing experience with fibrin sealant (see ADVERSE REACTIONS, Post-MarketingExperience (6.2)). In specific cases, these reactions have progressed to become life-threatening. Such reactions may especially be seen if ARTISS is applied repeatedly over time or in the same setting, or if systemic aprotinin has been administered previously; however, these reactions may also occur in patients receiving ARTISS for the first time. Even if the first treatment was well tolerated, a subsequent administration of ARTISS or systemic aprotinin may not exclude the occurrence of an allergic reaction. Symptoms associated with allergic anaphylactic reactions include: flush, urticaria, pruritus, nausea, drop in blood pressure, tachycardia or bradycardia, dyspnea, severe hypotension and anaphylactic shock.
Aprotinin is included in ARTISS for its antifibrinolytic properties. Aprotinin, a monomeric polypeptide, is known to be associated with anaphylactic reactions. Even in the case of strict local application of aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure (see CONTRAINDICATIONS, Aprotinin Hypersensitivity (4.2)).
Discontinue administration of ARTISS in the event of anaphylactic/-oid or hypersensitivity reactions. Remove the already applied, polymerized product from the surgical field. Mild reactions can be managed with antihistamines. Severe reactions and reactions involving hypotension require immediate resuscitative intervention.
5.2 Air or Gas Embolism
Air or gas embolism has occurred with the use of spray devices employing pressure regulator to administer fibrin sealants. This event appears to be related to the use of the spray device at higher than recommended pressures and in close proximity to the tissue surface.
When applying ARTISS using a spray device, be sure to use the pressure within the pressure range recommended by the spray device manufacturer. In the absence of a specific recommendation avoid using pressure above 20-25 psi. Do not spray closer than the distance recommended by the spray device manufacturer. In the absence of a specific recommendation avoid spraying closer than 10-15 cm from the surface of the tissue. When spraying ARTISS, changes in blood pressure, pulse, oxygen saturation and end tidal CO2 should be monitored because of the possibility of occurrence of air or gas embolism. When using the Easyspray device, or an equivalent spray device cleared by FDA, use the pressure within the pressure range recommended by the spray device manufacturer. Spray ARTISS only on to visible application sites.
5.3 Application Precautions
The sealer protein and thrombin solutions can be denatured by alcohol, iodine or heavy metal ions (e.g. antiseptic solutions). If any of these substances have been used to clean the wound area, the area must be thoroughly rinsed before application of ARTISS and made as dry as possible.
Apply ARTISS as a thin layer. Excessive clot thickness may delay the natural wound healing process.
5.4 Infection Risk from Human Plasma
ARTISS is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses. Despite these measures, such products can still potentially transmit disease. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses or other pathogens. All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Baxter Healthcare Corporation, telephone # 1-866-888-2472.
Some viruses, such as parvovirus B19, are particularly difficult to remove or inactivate at this time. Parvovirus B19 most seriously affects pregnant women (fetal infection), immune-compromised individuals or individuals with an increased erythropoiesis (e.g., hemolytic anemia) (see USE IN SPECIFIC POPULATIONS, Pregnancy (8.1)and PATIENT COUNSELING INFORMATION (17)).
6 ADVERSE REACTIONS
The most frequent (≥ 1% of clinical study subjects) adverse reactions with the use of ARTISS were: skin graft failure, hematoma and pruritus in burn studies, and hematoma/seroma in rhytidectomy studies.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The burn and rhytidectomy surgery studies were prospective, randomized, controlled, multicenter clinical studies with a total of 298 subjects. In each study the subject served as its own control. All subjects treated have been included into the safety analysis. See CLINICAL STUDIES (14) for outcome.
The data described in Table 2 reflects the exposure to ARTISS in the 4 burn and rhytidectomy surgery studies:
| Burn | Rhytidectomy | |||
| Parameter | Preliminary Study | Confirmatory Study | Preliminary Study | Confirmatory Study |
| Sample size (N) | 40 | 138 | 45 | 75 |
|
Gender F (%) / M (%) |
11 (27.5%) 29 (72.5%) |
44 (31.9%) 94 (68.1%) |
42 (93.3%) 3 (6.7%) |
71 (94.7%) 4 (5.3%) |
| Age Range (years) | 6 – 55 | 1 – 63 | 43 – 70 | 40 - 71 |
|
Volume applied (Mean ± SD) (Range in mL) |
2.9 ± 1.64 (Range: 1.0 - 10.8) |
2.7 ± 1.9 (Range: 0.2 - 12.0) |
2.32 ± 0.95 (Range: 0.80 - 4.0) |
2.58 ± 1.17 (Range: 0.60 - 4.0) |
Adverse reactions in the burn studies occurring in greater than 1% of subjects treated with ARTISS were skin graft failure (3%), hematoma (1%) and pruritus (1%) [n=178].
Adverse reactions in the facial rhytidectomy studies occurring in greater than 1% of subjects treated with ARTISS were hematoma/seroma (4%) [n = 120].
In the facial rhytidectomy studies, three subjects experienced serious adverse events (experiences). Two were local: wound abscess on the ARTISS treated side of the face that was recognized on postoperative day 14 and was treated by operative incision and drainage; and a case of basal cell carcinoma on the SoC treated side of the face. A third subject experienced dehydration on the second postoperative day.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of ARTISS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Fatal air embolism has been reported with misapplication of fibrin sealants administered with a pressurized spray device.
The following adverse reactions have been reported in post-marketing experience with another Baxter fibrin sealant that could reasonably be expected to occur with ARTISS:
Immune system disorders: anaphylactic responses, hypersensitivity
Cardiac disorders: bradycardia, tachycardia
Respiratory, thoracic and mediastinal disorders: dyspnea
Gastrointestinal disorders: nausea
Skin and subcutaneous tissue disorders: urticaria
General disorders and administration site conditions: flushing, impaired healing, edema, pyrexia
Injury, poisoning and procedural complication: seroma
7 DRUG INTERACTIONS
Oxycellulose containing preparations may reduce the efficacy of ARTISS and should not be used as carrier materials. No interaction studies have been performed.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with ARTISS. It is also not known whether ARTISS can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Some viruses, such as parvovirus B19, are particularly difficult to remove or inactivate at this time. Parvovirus B19 most seriously affects pregnant women (fetal infection). ARTISS should be given to a pregnant woman only if deemed medically necessary.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ARTISS is administered to nursing mothers.
8.4 Pediatric Use
In two clinical trials utilizing ARTISS to adhere autologous skin grafts to surgically prepared wound beds resulting from burns, the efficacy and safety of ARTISS in 38 pediatric subjects (27 subjects ages 1 through 10 years and 11 subjects ages 11 through 16 years) were not different from an adult population
10 OVERDOSAGE
To avoid the formation of excess granulation tissue and to ensure gradual absorption of the polymerized fibrin sealant, apply only a thin layer of ARTISS (see DOSAGE AND ADMINISTRATION, Method of Application (2.3)).
11 DESCRIPTION
ARTISS [Fibrin Sealant (Human)] is a two-component fibrin sealant made from pooled human plasma.
Sealer Protein (Human)
Sealer Protein (Human) is a sterile, non-pyrogenic, vapor-heated and solvent/detergent treated preparation made from pooled human plasma. Sealer Protein (Human) is provided either as a freeze-dried powder [Sealer Protein Concentrate (Human)] for reconstitution with Fibrinolysis Inhibitor Solution (Synthetic) or as a frozen liquid solution pre-filled into one side of a dual-chambered syringe (1). The active ingredient in Sealer Protein (Human) is fibrinogen. A Fibrinolysis Inhibitor, Aprotinin (Synthetic) is included in the Sealer Protein (Human) component to delay fibrinolysis. Aprotinin (Synthetic) is manufactured by solid phase synthesis from materials completely of non-human/non-animal origin.
Thrombin (Human)
Thrombin (Human) is a sterile, non-pyrogenic, vapor-heated and solvent/detergent treated preparation made from pooled human plasma. Thrombin (Human) is also provided either as a freeze-dried powder for reconstitution with Calcium Chloride Solution or as a frozen liquid solution pre-filled into one side of a dual-chambered syringe (2).
Sealer Protein (Human) and Thrombin (Human) are made from pooled human plasma collected at US licensed collection centers. The vapor heat and solvent/detergent treatment steps used in the manufacturing process have been shown to be capable of significant viral reduction. No procedure, however, has been shown to be completely effective in removing viral infectivity from derivatives of human plasma (see Viral Clearance below and WARNINGS/PRECAUTIONS, Infection Risk from Human Plasma (5.4).
Viral Clearance
The manufacturing procedure for ARTISS includes processing steps designed to further reduce the risk of viral transmission. In particular, vapor heating and solvent/detergent treatment processes are included in the manufacturing of Sealer Protein Concentrate and Thrombin. Validation studies were conducted using samples drawn from manufacturing intermediates for each of the two human plasma derived components. These samples were spiked with stock virus suspensions of known titers followed by further processing under conditions equivalent to those in the respective manufacturing steps. The stock virus suspensions represent HIV, HBV, HCV, HAV and Human Parvovirus B19.
The virus reduction factors (expressed as log10) of independent manufacturing steps are shown in Table 3 for each of the viruses tested:
| Reduction Factors for Virus Removal and/or Inactivation Sealer Protein Component |
|||||
| Manufacturing Step | Mean Reduction Factors [log10] of Virus Tested | ||||
| HIV-1* | HAV* | BVDV* | PRV* | MMV* | |
| Early Manufacturing Steps | n.d.† | n.d. | n.d. | n.d. | 2.7 |
| Solvent/Detergent Treatment | >5.3 | n.d. | >5.7 | >5.9 | n.d. |
| Vapor Heat Treatment | >5.5 | >5.6 | >5.7 | >6.7 | 1.2 |
| Overall Reduction Factor (ORF) | >10.8 | >5.6 | >11.4 | >12.6 | 3.9 |
| Reduction Factors for Virus Removal and/or Inactivation Thrombin Component |
|||||
| Mean Reduction Factors [log10] of Virus Tested | |||||
| Manufacturing Step | HIV-1 | HAV | BVDV | PRV | MMV |
| Thrombin precursor mass capture | 3.2 | 1.5 | 1.8 | 2.5 | 1.2 |
| Vapor Heat Treatment | >5.5 | >4.9 | >5.3 | >6.7 | 1.0 |
| Solvent/Detergent Treatment | >5.3 | n.d. | >5.5 | >6.4 | n.d. |
| Ion Exchange Chromatography | n.d. | n.d. | n.d. | n.d. | 3.6 |
| Overall Reduction Factor (ORF) | >14.0 | >6.4 | >12.6 | >15.6 | 5.8 |
In addition, Human Parvovirus B19 was used to investigate the upstream Thrombin precursor mass capture step, the Sealer Protein early manufacturing steps and the Thrombin and Sealer Protein vapor heating steps. Using quantitative PCR assays, the estimated log reduction factors obtained were 1.7 and 3.4 for the Thrombin precursor mass capture step and Sealer Protein early manufacturing steps and >4 / 1.0 for the Thrombin / Sealer Protein vapor heating steps, respectively.
See DOSAGE FORMS AND STRENGTHS (3).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Upon mixing Sealer Protein (Human) and Thrombin (Human), the two components mimic the final stage of the blood coagulation cascade. Soluble fibrinogen is transformed into fibrin that adheres to the wound surface and to the skin flap or graft to be affixed. Due to the low thrombin concentration, initial polymerization of ARTISS will take up to 60 seconds. The fibrin clot continues to strengthen for up to 2 hours after application.
Spray application of ARTISS over the wound bed provides full surface adherence of skin flaps and grafts. Full surface adherence minimizes areas of dead space between the wound bed and applied tissues. Elimination of dead space prevents shear irritation upon movement as well as reduces the void space under the skin that can host fluid build-up.
12.2 Pharmacodynamics
Thrombin is a highly specific protease that transforms the fibrinogen contained in Sealer Protein (Human) into fibrin (see Pharmacokinetics (12.3)).
Fibrinolysis Inhibitor, Aprotinin (Synthetic), is a polyvalent protease inhibitor that prevents premature degradation of fibrin. Free Aprotinin and its metabolites have a half-life of 30 to 60 minutes and are eliminated by the kidney. Preclinical studies with different fibrin sealant preparations simulating the fibrinolytic activity generated by extracorporeal circulation in patients during cardiovascular surgery have shown that incorporation of aprotinin in the product formulation increases resistance of the fibrin sealant clot to degradation in a fibrinolytic environment.
14 CLINICAL STUDIES
14.1 Burns (grafts)
ARTISS was investigated for adherence of split thickness sheet skin grafts in burn patients in a prospective, randomized, controlled, evaluator- blinded, multicenter clinical study. In each of the 138 patients, two comparable test sites were identified after burn wound excision. Skin grafts were adhered at one test site using ARTISS, and at the other test site using staples (control). The study product was applied once to the wound bed of the allocated test site during skin grafting surgery.
The mean ± SD estimated total body surface area (TBSA) for all burn wounds was 13.6 ± 9.2%. The mean ± SD estimated TBSA requiring skin grafting was 8.0 ± 6.9%. The mean ± SD estimated TBSA for ARTISS test sites was 1.7 ± 0.8% and for the stapled test sites was 1.7 ± 0.7%. Burn wound thickness was classified as full thickness in 106 (76.8%) of the 138 treated subjects, and partial thickness in 32 (23.2%) subjects.
The safety population contained all 138 treated subjects; however, 11 subjects did not have an available primary endpoint assessment, leaving a modified intent-to-treat (ITT) set of 127 patients. Complete wound closure by Day 28 was achieved in 43.3% of the ARTISS test sites and 37.0% of the stapled test sites in the 127 ITT patients. Wound closure at Day 28 was complete at 72% of the ARTISS and staples test sites for the 1-?6 years old group (N=18), at 32% of the ARTISS test sites and 26% of the staples test sites for the 7-18 years old group (N=19) and at 40% of the ARTISS test sites and 32% of the staples test sites for the greater then 18 years old group [ITT]. The lower limit of the 97.5% confidence interval of the difference between ARTISS and staples was –0.029. A similar result was obtained in the per protocol (PP) population: complete wound closure by Day 28 was achieved in 45.3% of the ARTISS test sites and 39.6% of the stapled test sites in the 106 PP patients. The lower limit of the 97.5% confidence interval of the difference between ARTISS and staples was –0.041. Therefore, ARTISS was found to be non-inferior to staples in the ITT and PP populations at the 97.5% one-sided level for complete wound closure by Day 28 because the lower limit of the confidence interval of the difference between ARTISS and staples success rates was greater than the predefined limit of –0.1.
14.2 Facial Rhytidectomy (flaps)
ARTISS was investigated for adherence of skin flaps in facial rhytidectomy surgeries during two prospective, randomized, controlled, multicenter clinical studies. Both the preliminary study investigating 45 subjects and the confirmatory study with 75 subjects had a split-face design in which 1 side of the face was treated with ARTISS as an adjunct to the standard of care (SoC) and the other side received SoC only, which was closure of the flap by means of staples and suturing only; therefore each subject participated in both arms (ARTISS and SoC).
Primary endpoint of the confirmatory study conducted in 75 subjects was the total drainage volume collected from each side of the face at 24 h (±4 h) post surgery. Occurrence of hematoma and seroma on each side of the face, comparison of edema between the 2 sides of the face, changes in skin sensitivity from baseline on each side of the face and subject preference were evaluated as secondary endpoints.
In both studies, a standardized drain was placed in each side of the face prior to the flap closure and drainage volume from both sides of the face from all subjects was compared. Pressure dressings were not allowed.
The results for the primary endpoint of the confirmatory study are presented in Table 4a below.
| Clinical Study (n= 75) | Mean ± SD Drainage (mL) ARTISS Side of the Face | Mean ± SD Drainage (mL) SoC Side of the Face | p-Value |
| Confirmatory study | 7.7 ± 7.4 | 20.0 ± 11.3 | < 0.0001 |
A statistically significant difference in drainage volumes was observed, favoring the side of the face treated with ARTISS.
Drainage volumes at 24 h post operatively for each side of the face reported as secondary endpoint in the preliminary study are presented in Table 4b below.
| Clinical Study (n = 45) | Mean ± SD Drainage (mL) ARTISS Side of the Face | Mean ± SD Drainage (mL) SoC Side of the Face |
| Preliminary study | 11.5 ± 13.7 | 26.8 ± 24.0 |
An integrated analysis of the occurrence of hematoma/seroma in all 120 subjects across two studies was performed. A comparison of the proportion of subjects experiencing a hematoma/seroma exclusively on the ARTISS-treated side or on the SoC side of the face is presented in Table 5 below.
| Clinical Study |
ARTISS n (%) |
SoC n (%) |
Both Sides of Face n (%) |
Total n (%) |
| Preliminary study | 0 | 9 (20%) | 0 | 9 (20%) |
| Confirmatory study | 2 (2.7%) | 5 (6.7%) | 3 (4%) | 10 (13.3%) |
16 HOW SUPPLIED/STORAGE AND HANDLING
ARTISS is supplied in the following pack sizes and presentations:
| Pack Size | NDC Number | ||
| ARTISS Kit (Freeze-Dried) | ARTISS Kit (Freeze-Dried) with DUPLOJECT System | ARTISS Pre-Filled Syringe (Frozen) with DUO Set | |
| 2 mL | 0944-4351-03 | 0944-4351-04 | 0944-8503-02 |
| 4 mL | 0944-4351-07 | 0944-4351-08 | 0944-8503-04 |
| 10 mL | 0944-4351-11 | 0944-4351-12 | 0944-8503-10 |
See DOSAGE FORMS AND STRENGTHS (3).
Storage
Store ARTISS in original carton to protect from light. Do not use after the expiration date. Discard if packaging of any components is damaged.
ARTISS Kit (Freeze-Dried)
Store at 2°C to 25°C. Avoid freezing. After reconstitution, the product must be used within 4 hours. Reconstituted solutions must not be refrigerated or frozen.
ARTISS Pre-filled Syringe (Frozen)
| Long term: | Store at ≤ -20°C. |
| Short term: | Room Temperature Thawing: Unopened pouches, thawed at room temperature, may be stored for up to 14 days at room temperature (15-25°C) after removal from the freezer. |
| Quick Thawing: Maintain the product at 33-37°C until use. If the product is removed from original pouch or warmed to 33-37°C it must be used within 12 hours. | |
| Do not refrigerate or re-freeze after thawing. Do not microwave. |
Do not use after the expiration date. Discard if packaging of any components is damaged.
17 PATIENT COUNSELING INFORMATION
Inform patients that ARTISS is made from human plasma and discuss the risks and benefits with the patient.
Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased red blood cell turnover. Instruct patients to consult their physician if symptoms of B19 virus infection appear (fever, drowsiness, chills and runny nose followed about two weeks later by a rash and joint pain (see USE IN SPECIFIC POPULATIONS, Pregnancy (8.1)).
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
US License No. 140
This product is covered under US Patent Nos. 5,962,405 and 6,579,537.
Baxter, Artiss, Easyspray, Fibrinotherm and Duploject are trademarks of Baxter International
Principal Display Panel
ARTISS 4 mL kit unit carton
NDC 0944-4351-07
4 mL
Kit
Fibrin Sealant (Human)
ARTISS
Contents:
Sealer Protein Concentrate (Human). Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Sealer Protein Solution
Fibrinolysis Inhibitor Solution (Synthetic), Sterile, 3000 KIU of Aprotinin/mL
Thrombin (Human), Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Thrombin Solution 4 IU/mL
Calcium Chloride Solution. Sterile, 40 µmol/mL
FOR TOPICAL USE ONLY
NOT FOR INJECTION
Do not use on individuals with a know hypersensitivity to aprotinin. The risk and benefits of this product should be discussed with the patient. Read the full prescribing information before use.
For single use only.
No preservative
Latex free
Rx Only
Baxter
ARTISS 4 mL kit sleeve
NDC 0944-4351-08
4 mL
Kit
Fibrin Sealant (Human)
ARTISS
Contents:
Sealer Protein Concentrate (Human). Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Sealer Protein Solution
Fibrinolysis Inhibitor Solution (Synthetic), Sterile, 3000 KIU of Aprotinin/mL
Thrombin (Human), Vapor Heated, Solvent/Detergent Treated, freeze dried, sterile, for 2 mL of Thrombin Solution 4 IU/mL
Calcium Chloride Solution. Sterile, 40 µmol/mL
Also includes: DUPLOJECT System for ARTISS [Fibrin Sealant (Human)] 2mL/4mL
FOR TOPICAL USE ONLY
NOT FOR INJECTION
Do not use on individuals with a know hypersensitivity to aprotinin. The risk and benefits of this product should be discussed with the patient. Read the full prescribing information before use.
For single use only.
No preservative
Latex free
Rx Only
Baxter

Sealer Protein Concentrate (Human) vial label
NDC 0944-7112-02
Sealer Protein Concentrate (Human)
Baxter
Vapor Heated, Solvent/Detergent Treated, Freeze-Dried, Sterile
Reconstitute with 2 mL of Fibrinolysis Inhibitor Solution (Synthetic)
Vial contains a stirrer to facilitate reconstitution.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Use within 4 hours of reconstitution.
Read enclosed directions prior to use.
NOT FOR INJECTION.
Store at 2ºC to 25ºC.
Rx only

Thrombin (Human) vial label
NDC 0944-7344-02
Thrombin (Human)
Baxter
Vapor Heated, Solvent/Detergent Treated, Freeze-Dried, Sterile
Reconstitute with 2 mL of Calcium Chloride Solution
Concentration: 4 IU/mL
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx Only

Calcium Chloride Solution vial label
NDC 0944-7401-02
Calcium Chloride Solution
Baxter
Sterile For reconstitution of freeze-dried Thrombin (Human), Contents: 2 mL
Concentration: 40 µmol/mL
NOT FOR INJECTION. Store at 2°C to 25°C.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx Only

Fibrinolysis Inhibitor Solution (Synthetic) vial label
NDC 0944-7201-01
Fibrinolysis Inhibitor Solution (Synthetic)
Baxter
Sterile For reconstitution of freeze-dried Sealer Protein Concentrate (Human), Contents: 1 mL
Concentration: 3000 KIU of Aprotinin/mL
NOT FOR INJECTION. Store at 2°C to 25°C.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx Only

ARTISS 2 mL and 4 mL DUPLOJECT unit carton
DUPLOJECT 2 mL / 4 mL
Preparation and Application System for ARTISS [Fibrin Sealant (Human)]
For the preparation and application of ARTISS [Fibrin Sealant (Human)] 2 mL/4mL Kit.
STERILE - SINGLE PATIENT USE ONLY - DO NOT RESTERILIZE
Use separate devices for reconstitution of Sealer Protein Concentrate and Thrombin.
Rx only
Baxter

ARTISS Frozen 4 mL unit carton
NDC 0944-8503-04
4 mL
Fibrin Sealant (Human)
ARTISS
Frozen
TOPICAL USE ONLY
DO NOT INJECT
Contents:
Pre-filled syringe containing:
Sealer Protein Solution (1): 2 mL, sterile
Sealer Protein (Human) Vapor Heated, Solvent /Detergent Treated
Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mL
Thrombin Solution (2): 2 mL, sterile
Thrombin (Human), 4 units/mL Vapor Heated, Solvent /Detergent Treated
Calcium Chloride, 40 µmol/mL
Do not use on individuals with a known hypersensitivity to aprotinin and/or any of the active substances or excipients.
The risks and benefits of this product should be discussed with the patient.
Read full prescribing information before use.
Store at -20°C (-4°F) or colder. Unopened pouches, thawed at room temperature, may be stored for up to 14 days at 15-25°C.
The product must be used within 12 hours after warming to 33-37°C or removal form original pouches.
Do not refrigerate, microwave, or re-freeze.
For single use only
No preservative
Latex free
Rx Only
Contents were sterilized and package under aseptic conditions.

ARTISS Frozen 4 mL pouch label
NDC 0944-8503-04
Fibrin Sealant (Human)
ARTISS 4 mL
Frozen
Baxter
Contents:
Pre-filled syringe containing:
Sealer Protein Solution (1): 2 mL, sterile
Sealer Protein (Human)
Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mL
Thrombin Solution (2): 2 mL, sterile
Thrombin (Human), 4 units/mL
Calcium Chloride, 40 µmol/mL
Read directions for thawing and application before use.
TOPICAL USE ONLY
DO NOT INJECT
Store at -20°C (-4°F) or colder. Unopened pouches, thawed at room temperature, may be stored for up to 14 days at 15-25°C.
Do not refrigerate, microwave, or re-freeze.
Rx Only
Contents were sterilized and package under aseptic conditions.
Baxter Healthcare Corporation
Westlake Village, CA 91362 USA
U.S. License No. 140
Rx Only
ARTISS 4 mL Syringe label
Baxter
Fibrin Sealant (Human)
ARTISS
4 mL
| ARTISS
fibrinogen human thrombin human kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxalta US Inc. (079887619) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Aktiengesellschaft | 300434670 | MANUFACTURE | |