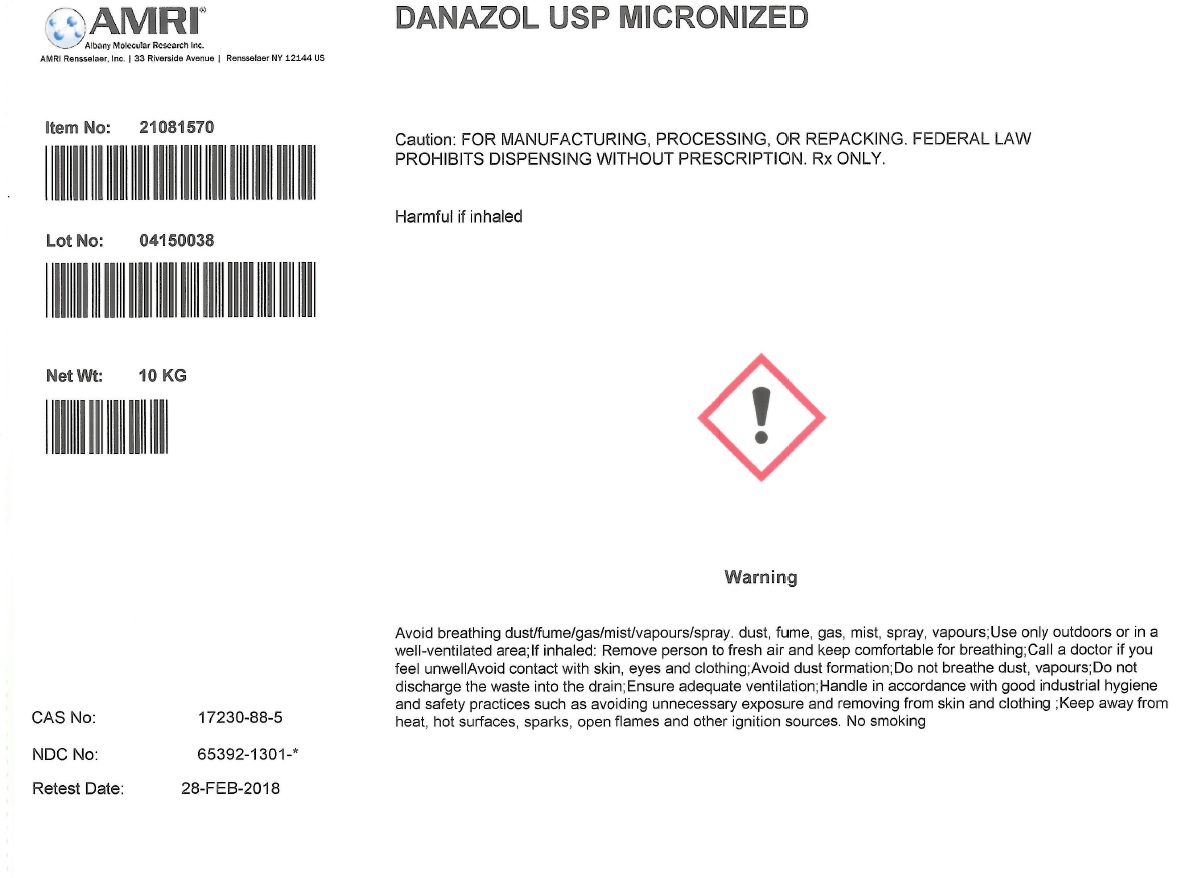
DANAZOL- danazol powder
AMRI Rensselaer, Inc.
----------
Danzol
| DANAZOL
danazol powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - AMRI Rensselaer, Inc. (124193793) |
| Registrant - AMRI Rensselaer, Inc. (124193793) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMRI Rensselaer, Inc. | 124193793 | api manufacture(65392-1301) | |
Revised: 5/2020
Document Id: a6407437-e99b-150b-e053-2995a90acced
Set id: 38152348-509c-42ae-e054-00144ff8d46c
Version: 4
Effective Time: 20200522
AMRI Rensselaer, Inc.