Label: SODIUM IODIDE I-131 kit
- NDC Code(s): 69208-000-00, 69208-003-15, 69208-003-25, 69208-003-35
- Packager: International Isotopes Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SODIUM IODIDE I-131 safely and effectively. See full prescribing information for SODIUM IODIDE I-131.
SODIUM IODIDE I-131 (for the preparation of sodium iodide I 131 capsules and solution), therapeutic, for oral use.
Initial U.S. Approval: 1971INDICATIONS AND USAGE
Sodium Iodide I-131 is a radioactive therapeutic agent indicated for the treatment of hyperthyroidism and selected cases of carcinoma of the thyroid. (1)
DOSAGE AND ADMINISTRATION
- The concentrated sodium iodide I 131 solution provided must be diluted. ( 2.2)
- See Full Prescribing Information for important administration instructions and dilution and preparation instructions for sodium iodide l 131 capsules or oral solution. ( 2.2, 2.4)
- The recommended dose is based on the thyroid gland uptake as well as the size of the gland:
- Treatment of Hyperthyroidism: Recommended dosage is 148 to 370 megabecquerels (MBq) [4 to 10 millicuries (mCi). ( 2.3)
- Treatment of Thyroid Carcinoma: Recommended dosage is 1,110 to 33,700 MBq (30 to 100 mCi). ( 2.3)
DOSAGE FORMS AND STRENGTHS
Vials: Sodium Iodide I-131 solution (with a radioconcentration of 37,000 MBq/mL (1000 mCi/mL) at the time of calibration) for the preparation of sodium iodide I-131 capsules, therapeutic or sodium iodide I-131 solution, therapeutic. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Radiation-induced thyroiditis may cause or worsen hyperthyroidism. Consider pre-treatment with anti-thyroid medications. ( 5.1)
- Multiple non-thyroid radiation toxicities, including hematopoietic suppression: Individualize dose and monitor for toxicity. ( 5.2)
- Fetal toxicity: May cause severe and irreversible hypothyroidism in the neonate. Verify absence of pregnancy before administering the product. ( 5.4, 8.1, 8.3)
- Radiation exposure to breast tissue with lactation: Sodium iodide I 131 concentrates in the breast of lactating women. Discontinue lactation 6 weeks prior to therapy. ( 5.5, 8.2)
ADVERSE REACTIONS
Common adverse reactions reported with therapeutic doses of sodium iodide I-131 include local swelling, radiation sickness, sialadenitis, salivary gland dysfunction, bone marrow depression, lacrimal gland dysfunction, hypothyroidism, hyperthyroidism, thyrotoxic crisis.( 6)
To report SUSPECTED ADVERSE REACTIONS, contact International Isotopes Inc. at 1-800-699-3108 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2.1 Radiation Safety
2.2 Important Administration Instructions
2.3 Recommended Dosage and Administration
2.4 Dilution and Preparation Instructions
2.5 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Radiation-induced Thyroiditis
5.2 Radiation-induced Toxicities
5.3 Hypersensitivity Reactions
5.4 Embryo-Fetal Toxicity
5.5 Increased Radiation Exposure to Breast Tissue with Lactation
5.6 Transient Infertility
5.7 Risk of Radiation Exposure
5.8 Risk of Decreased Effectiveness of Therapy
6 ADVERSE REACTIONS
6.1 Postmarketing Experience
7 DRUG INTERACTIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
DOSAGE & ADMINISTRATION
2.1 Radiation Safety
- Sodium Iodide I-131 is a radioactive drug. Handle with appropriate safety measures to minimize radiation exposure to the patient and healthcare workers [see Warnings and Precautions (5.7)]:
- Use only by, or under the direction of, physicians who are qualified by specific training and experience in the safe use and handling of radioactive materials, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radiopharmaceuticals.
- Use waterproof gloves when handling and administering the product.
- Maintain adequate shielding during the life of the product.
- Measure patient dose with a suitable radioactivity calibration system immediately prior to administration.
2.2 Important Administration Instructions
- Do not directly administer the concentrated Sodium Iodide I-131 solution provided to patients. The concentrated sodium iodide I 131 solution must be diluted and prepared prior to administration [see Dosage and Administration (2.4)] .
- Obtain a pregnancy test in females of reproductive potential prior to administration to verify the absence of pregnancy [see Contraindications (4) and Use in Specific Populations (8.1,8.3)] .
- Instruct patients to fast at least 2 hours before and 2 hours after administration to ensure absorption.
- Instruct patients to hydrate before and after administration of sodium iodide I-131 and to void frequently to enhance urinary elimination of the radioiodide that is not absorbed by the thyroid gland [see Warnings and Precautions (5.2)] .
- Instruct patients to maintain a low-iodide diet two weeks prior to radioiodide administration and continue for several days during the uptake or imaging process [see Warnings and Precautions (5.8) and Drug Interactions (7)] .
- Instruct patients to discontinue the anti-thyroid therapy three days before administration of sodium iodide I 131 [see Warnings and Precautions (5.8) and Drug Interactions (7)] .
- For patients with a history of renal impairment, evaluate renal function for therapeutic planning and consider dosimetry [see Use in Specific Populations (8.6)] .
- Obtain a complete blood count within one month of therapy. If patients show leukopenia or thrombocytopenia, dosimetry should be used to determine a safe sodium iodide I 131 activity, while delivering less than 2 Gy to the bone marrow [see Warnings and Precautions (5.2)] .
2.3 Recommended Dosage and Administration
Individualization of Therapy
The recommended dose for orally administered sodium iodide I 131 capsules or solution is based on the thyroid gland uptake as well as the size of the gland. Thyroidal uptake and size should be determined by the physician prior to treatment and may be useful in calculating the therapeutic dose to be administered to the individual patient.Treatment of Hyperthyroidism
The recommended dose is 148 to 370 MBq (4 to 10 mCi) administered orally. Toxic nodular goiter may require a larger dose.Treatment of Thyroid Carcinoma
The recommended dose is 1100 to 3700 MBq (30 to 100 mCi) administered orally. For subsequent ablation of metastases, the recommended dose is 3700 to 7400 MBq (100 to 200 mCi) administered orally.2.4 Dilution and Preparation Instructions
Drug Handling
- Wear waterproof gloves throughout the entire handling and administration procedure.
- Make all transfers of radioactive solutions with an adequately shielded syringe or remote handling equipment and maintain adequate shielding around the vial during the useful life of the radioactive product.
Preparation of Dilute Sodium Iodide I 131 Solution
1. Using the calibration date and radionuclidic concentration on the label of the product vial, calculate the required volume to produce the necessary dose in MBq or mCi.
2. Using a shielded syringe, remove the required volume.
3. Using the shielded syringe, transfer the required volume to a suitably shielded receiving vial.
4. Add diluent to the receiving vial to produce a final dose of the desired volume.
5. The recommended diluent is Purified Water USP containing 0.2 % sodium thiosulfate USP as a reducing agent. Acidic diluents should not be used as they may cause the pH to drop below 7.5 and stimulate the volatilization of Iodine I-131 hydriodic acid.
6. Present the dose in a shielded container for administration to the patient with a straw.
Preparation of Sodium Iodide I 131 Capsules
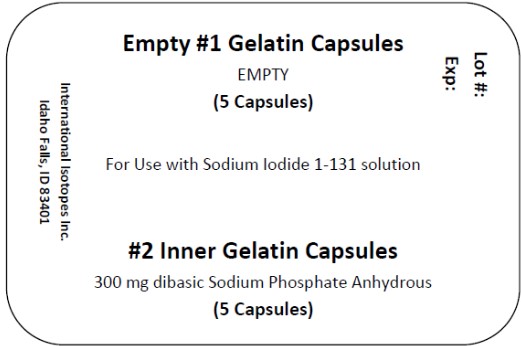
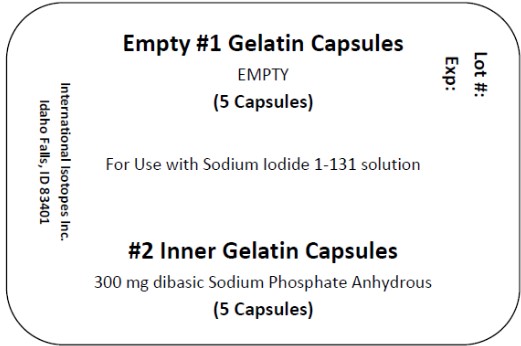
1. Use one large gelatin capsule and one small gelatin capsule for each dose to be prepared. Each large capsule is empty and each small capsule contains approximately 300 mg of dibasic sodium phosphate anhydrous as the absorbing buffer.
2. Using the calibration date and radionuclidic concentration on the label of the sodium iodide I-131 lead shield, calculate the required volume to produce the necessary dose in MBq or mCi.
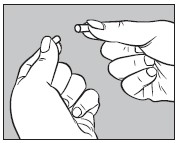
3. Open one large capsule by pulling apart the capsule into two pieces as illustrated below:
4. Insert an unopened small capsule into the bottom half of the empty large capsule as illustrated below: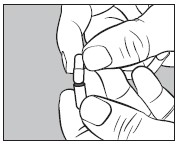
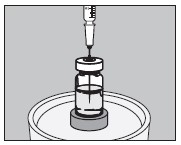
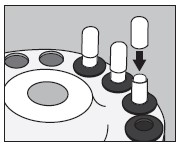
5. With an appropriate syringe, withdraw the required volume of sodium iodide I 131 Solution (maximum 150 microliters) from the vial as illustrated below:
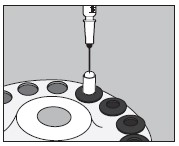
6. Inject into the center of the small capsule through the top as illustrated below:
7. Slip the upper half of the large capsule over the bottom half to completely cover the small capsule and push down gently until locked as illustrated below:
8. Measure the patient dose in a suitable radioactivity calibration system immediately prior to administration.
9. Prepared capsules may be stored in a suitable polypropylene container and place inside a lead pot until use, within seven days.
2.5 Radiation Dosimetry
- The biokinetic modeling and radiation dose distributions associated with thyroid uptake of iodide I 131 depend on dietary intake of stable iodide and presume normal production of thyroid hormone. Table 1 shows a range of uptake percentages in an average adult (73.7 kg reference model). Table 1 is not intended to be used for treatment planning.
- For a thyroid blocked from iodide uptake in the production of hormones, the effective half-life of iodide I 131 is approximately 1.4 hours; for "low" to "high" uptake, the effective half-life of I 131 ranges from approximately 80 to 90 hours.
Table 1 * - *
- Table 1 is not intended for treatment planning.
- †
- These columns are not applicable to estimate organ or effective doses in patients following thyroidectomy. In patients with thyroid cancer following thyroidectomy, organ and effective doses can be estimated from the "blocked"-thyroid-uptake values.
- ‡
- These values presume unimpeded production of thyroid hormone and may not be applicable to estimate thyroid dose and effective dose in patients who have had previous treatment with I 131 for hyperthyroidism
Absorbed dose per unit activity sodium iodide I 131 administered orally (mGy/MBq) in adult (73.7-kg reference model) Organ Thyroid uptake of I 131 (% administered activity A 0)
24 h after oral administrationBlocked thyroid
(0% A 0)Low uptake †
(16% A 0)Medium uptake †
(26% A 0)High uptake †
(36% A 0)Adrenals 0.044 0.051 0.055 0.059 Bone surfaces 0.030
0.089 0.12 0.16 Brain 0.021 0.093 0.13 0.17 Breast 0.020 0.038 0.048 0.058 Gallbladder wall 0.037 0.043 0.046 0.049 Gastrointestinal tract Esophagus 0.024 0.10 0.14 0.19 Stomach wall 0.87 0.77 0.71 0.66 Small intestine wall 0.035 0.033 0.032 0.032 Colon wall 0.14 0.14 0.14 0.14 (Upper large intestine wall) 0.12 0.12 0.12 0.12 (Lower large intestine wall) 0.17 0.17 0.17 0.16 Heart wall 0.062 0.089 0.10 0.12 Kidneys 0.27 0.27 0.27 0.27 Liver 0.050 0.093 0.12 0.14 Lungs 0.053 0.10 0.13 0.15 Muscles 0.026 0.084 0.12 0.15 Ovaries 0.038 0.037 0.036 0.035 Pancreas 0.060 0.064 0.066 0.068 Red marrow 0.031 0.072 0.095 0.12 Salivary glands 0.27 0.22 0.19 0.16 Skin 0.019 0.043 0.057 0.071 Spleen 0.064 0.069 0.072 0.075 Testes 0.025 0.024 0.023 0.22 Thymus 0.024 0.10 0.14 0.19 Thyroid 2.2 280 ‡ 430 ‡ 580 ‡ Urinary bladder wall 0.54 0.45 0.39 0.34 Uterus 0.045 0.042 0.040 0.038 Remaining organs 0.029 0.084 0.11 0.15 Effective dose per administered activity (mSv/MBq) 0.28 14 ‡ 22 ‡ 29 ‡ -
3 DOSAGE FORMS AND STRENGTHS
Sodium Iodide I-131 solution componentis available in customer ordered vials available in 1, 2 and 3 mL V-Vials containing colorless, aqueous concentrated Sodium Iodide I-131 Solution at a strength of 37,000 MBq/mL (1000 mCi/mL) at time of calibration for preparation of sodium iodide I-131 capsules, therapeutic or sodium iodide I-131 solution, therapeutic. Large empty gelatin capsules and small gelatin capsules containing approximately 300 mg of dibasic sodium phosphate anhydrous as the absorbing buffer are supplied along with the Sodium Iodide I-131 solution for the preparation of sodium iodide I-131 capsules, therapeutic.
Table 2. Sodium Iodide I-131 Dispensing Concentrate Solution *At time of calibration Vial Type (Stopper) mCi I-131/mL Maximum Volume (mL) 1 mL V-Vial (13 mm) 1000 0.75 2 mL V-Vial (13 mm) 1000 1.50 3 mL V-vial (20 mm) 1000 2.25 -
4 CONTRAINDICATIONS
Sodium Iodide I-131 is contraindicated in:
- Patients with vomiting and diarrhea [see Warning and Precautions (5.7)] .
- Patients with thyroid malignancies shown to have no iodide uptake, which include the majority of medullary or anaplastic carcinomas.
- Patients receiving concurrent anti-thyroid therapy [see Warning and Precautions (5.1) and Drug Interactions (7)] .
- Pregnancy [see Warnings and Precautions (5.4), see Use in Specific Populations (8.1)] .
- Lactation [see Warnings and Precautions (5.5)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Radiation-induced Thyroiditis
Sodium iodide I 131 may cause thyroiditis with release of thyroid hormone, which may aggravate hyperthyroidism and thyrotoxic cardiac disease [see Adverse Reactions (6)] . When treating hyperthyroidism, consider pre-treatment anti-thyroid medication to help deplete the thyroid hormone. Discontinue the anti-thyroid therapy three days before administration of sodium iodide I 131 [see Drug Interactions (7)] . Consider a beta-blocker pre or post-treatment to minimize the risk of hyperthyroidism and thyroid storm.
The thyroiditis may cause gland enlargement resulting in tenderness and swelling of the neck, pain on swallowing, sore throat, and cough; which may occur approximately the third day after sodium iodide I 131 administration. Consider management with pain-reliever or anti-inflammatory medications.
5.2 Radiation-induced Toxicities
Sodium Iodide I 131 may cause radiation induced toxicities [see Adverse Reactions (6)] :
- Dose-dependent fatalities (bone marrow suppression, malignancy).
- Dose-dependent hematopoietic suppression which manifests as a transient thrombocytopenia or neutropenia 3-5 weeks following sodium iodide I 131 administrations, may lead to increased susceptibility to infections or bleeding.
- Salivary gland toxicity: sialadenitis, xerostomia.
- Lacrimal gland toxicity: conjunctivitis, xerophthalmia, and epiphora.
- Increased risk of developing new solid tumors and leukemias.
Obtain a complete blood count within one month of therapy. If patients show leukopenia or thrombocytopenia, dosimetry should be used to determine a safe sodium iodide I 131 activity, while delivering less than 2 Gy to the bone marrow.
Advise good hydration for one week following sodium iodide I 131 administration and stimulate salivary flow via a sialagogue (e.g. sugar-free candy or gum, pilocarpine, and ascorbic acid) to reduce radiation exposure to the salivary glands.
Advise patients to void frequently after administration of radioiodide to enhance excretion.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions including anaphylaxis may occur in patients who receive sodium iodide I 131. Although iodide is not considered an allergen, hypersensitivity reactions may occur in relation with excipients or chemical component of the capsule, such as sodium thiosulfate. Obtain and document an allergy history, particularly a sulfite allergy. Emergency resuscitation equipment and personnel should be immediately available [see Adverse Reactions (6)] .
5.4 Embryo-Fetal Toxicity
Sodium Iodide I-131 Solution is contraindicated in pregnancy because sodium iodide I-131 crosses the placenta and fetal exposure can lead to neonatal hypothyroidism. Multiple reports in the published literature describe hypothyroidism in the neonates following in utero exposure to sodium iodide I-131. Some cases of neonatal hypothyroidism were severe and irreversible. Verify pregnancy status of females of reproductive potential prior to initiating Sodium Iodide I-131 Solution treatment. Advise females and males of reproductive potential to use effective contraception during treatment with Sodium Iodide I-131 Solution and for at least six months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.5 Increased Radiation Exposure to Breast Tissue with Lactation
Sodium Iodide I-131 Solution is contraindicated in lactating women because sodium iodide I-131 concentrates in the breast via the increased expression of the sodium iodide symporter in breast tissue with lactation. The literature describes moderate to marked radioiodine uptake in the breast tissue for 5 to 32 weeks post cessation of breast feeding. Advise lactating women to discontinue breast feeding at least 6 weeks prior to administration of sodium iodide I-131 to allow sufficient time for involution to occur and to avoid excess concentration of sodium iodide I-131 in breast tissue. Consider administration of drugs to suppress lactation. Consider diagnostic scintigraphy before administration of sodium iodide I-131 to assess the persistence of uptake by breast tissue. If sodium iodide I-131 is administered in the postpartum period, the lactating mother should not breastfeed the infant [see Use in Specific Populations (8.2)] .
5.6 Transient Infertility
Transient dose-related impairment of testicular function in men and transient ovarian insufficiency in women has been reported after sodium iodide I-131 therapy. Sperm banking for men may be considered prior to administration of Sodium Iodide I-131 Solution for thyroid carcinoma [see Use in Specific Populations (8.3)] .
5.7 Risk of Radiation Exposure
Household Contacts
Instruct patients to follow radiation safety precautions after receiving Sodium Iodide I-131 to minimize the radiation contamination of other persons or the environment. Patients should avoid close contact with others, especially pregnant women and children, and take care to avoid contamination of other persons or the environment with body fluids.Patients and Healthcare Providers
Sodium Iodide I-131 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk of cancer. Follow safe handling and administration to minimize radiation exposure to the patient and healthcare providers.5.8 Risk of Decreased Effectiveness of Therapy
Certain food or drugs may alter the thyroid uptake of sodium iodide I 131 and diminish its effectiveness. Recent intake of stable iodide in any form, or the use of thyroid or anti-thyroid drugs may diminish thyroid uptake of sodium iodide I 131 [see Drug Interactions (7)] .
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described below and elsewhere in labeling:
- Radiation-induced Thyroiditis [see Warnings and Precautions (5.1)] .
- Radiation-induced Toxicities [see Warnings and Precautions (5.2)].
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)].
- Embryo-Fetal Toxicity[see Warnings and Precautions (5.4), Use in Specific Population (8.1)].
- Increased Radiation Exposure to Breast Tissue with Lactation [see Warnings and Precautions (5.5), Use in Specific Populations (8.2)].
- Transient Infertility [see Warnings and Precautions (5.6), Use in Specific Population (8.3)].
- Radiation Exposure to Other Individuals [see Warnings and Precautions (5.7)].
- Risk of Decreased Effectiveness of Therapy [see Warnings and Precautions (5.8)].
6.1 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of sodium iodide I-131 (Table 3). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 3 Postmarket Adverse Reactions by System Organ Class
System Organ Class*
Symptoms*
Cardiac disorders
Chest pain, tachycardia
Congenital, familial and genetic disorders
Chromosomal abnormalities, congenital hypothyroidism
Endocrine disorders
Hyperthyroidism, hypoparathyroidism, hypothyroidism, thyrotoxic crisis
Eye disorders
Lacrimal gland dysfunction
Gastrointestinal disorders
Gastritis, nausea, salivary gland dysfunction, sialadenitis, vomiting
General disorders and administration site conditions
Local swelling of thyroid or sites of iodide avid tumor
Hematologic and lymphatic disorders including fatalities
Anemia, blood dyscrasia, bone marrow depression, leukopenia, thrombocytopenia
Immune system disorders
Bronchospasm
Neoplasms benign, malignant and unspecified (including cysts and polyps)
Acute leukemia, solid cancer
Nervous system disorders
+Cerebral edema, headache
Respiratory, thoracic and mediastinal disorders
♦Pulmonary fibrosis, ♦radiation pneumonitis
Skin and subcutaneous tissue disorders
Hives, itching, rash
* In alphabetical order
+ In patients with iodide-avid brain metastases
♦ In patients with iodide-avid lung metastases
-
7 DRUG INTERACTIONS
- Concomitant use of bone marrow depressants may enhance the depression of the hematopoietic system caused by the use of large doses of sodium iodide I 131 [see Warnings and Precautions (5.2)] .
- Many drugs and iodide-containing foods interfere with the accumulation of radioiodide by the thyroid. Review the patients history, current medications, and recent diagnostic tests prior to the administration of sodium iodide I 131 [see Warnings and Precautions (5.8)] .
- Advise patients to maintain a low-iodide diet two weeks prior to radioiodide administration and continue for several days during the uptake or imaging process and to discontinue taking the following products before they undergo the procedure as shown in Table 4.
Table 4 Pharmaceuticals/OTCs/Agents Blocking Radioiodine Uptake Type of Medication Recommended time of withdrawal Thionamide medications
(e.g., propylthiouracil, methimazole, carbimazole)3 days Multivitamins containing iodide 10 days Natural or synthetic thyroid hormones
triiodothyronine
thyroxine2 weeks
4 weeks
Iodide-containing foods: iodized salt, dairy products, egg yolks, seafood, turkey and liver 2 weeks Kelp, agar, carrageenan, Lugol solution 3 weeks Saturated solution of potassium iodide 3 weeks Topical iodide
(e.g., surgical skin preparation)3 weeks Intravenous radiographic contrast agents
Water soluble
Lipophilic2 months
6 months
Amiodarone 6 months -
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Sodium Iodide I-131 is contraindicated in pregnancy because fetal exposure can lead to neonatal hypothyroidism, which in some cases is severe and irreversible [see Warnings and Precautions (5.4)] . Data from the published literature describe thyroid abnormalities after fetal exposure; including agenesis of the thyroid and hypothyroidism (see Data). No animal reproductive studies have been conducted.Clinical Considerations.
Fetal/ Neonatal Adverse Reactions
A fetus exposed to sodium iodide I 131 can develop neonatal hypothyroidism. Delay in diagnosis of neonatal hypothyroidism after exposure to sodium iodide I 131 in utero can result in severe sequelae such as decreased mental capacity and delayed bone age. Monitor thyroid function in any infant born after in utero exposure to sodium iodide I 131.Data
Human Data
Sodium iodide I 131 crosses the placenta and the fetal thyroid begins to concentrate iodide during the 10-12 th week of gestation. In literature reports of maternal exposures to sodium iodide I 131 at doses of 333 – 8325 MBq (9 – 225 mCi) during 4-26 weeks gestational age, the most common adverse outcomes were hypothyroid infants and children.8.2 Lactation
Risk Summary
Sodium Iodide I-131 Solution is contraindicated during lactation because I-131 concentrates in the breast during lactation via the increased expression of the sodium iodide symporter in breast tissue and can lead to hypothyroidism in the infant through breastfeeding. If sodium iodide I-131 is administered postpartum, breastfeeding should not be restarted for the remainder of the postpartum period. In addition, to minimize the absorbed radiation dose to the breast tissue, breastfeeding and breast-pumping should be discontinued for at least 6 weeks before administration of sodium iodide I-131 [see Warnings and Precautions (5.5)] . Infants exposed to sodium iodide I-131 through breast milk are at risk for development of hypothyroidism because sodium iodide I-131 is distributed into breast milk and may reach concentrations equal to or greater than concentrations in maternal plasma(see Data).Data
Published literature describes sodium iodide I-131 transfer into breast milk and uptake by the thyroid of the breastfed infant. The amount of sodium Iodide I-131 detected in the breast milk at 36 to 48 hours after administration is 1% to 27% of the injected dose (with injected doses between 1.1 MBq to 5,143 MBq).8.3 Females and Males of Reproductive Potential
Sodium Iodide I-131 is contraindicated in pregnancy because of the risk of fetal hypothyroidism [see Warnings and Precautions (5.4) and [see Use in Specific Populations (8.1)] .
Pregnancy Testing
Obtain a pregnancy test in females of reproductive potential and verify the absence of pregnancy before initiating treatment [see Dosage and Administration (2.2)].Contraception
Advise females and males of reproductive potential to use effective contraception during treatment with Sodium Iodide I-131 Solution and for at least six months after the last dose of Sodium Iodide I-131 Solution.Infertility
Females
Fertility may be impaired with Sodium Iodide I-131 Solution treatment. Transient amenorrhea and ovarian insufficiency have been observed after sodium iodide I-131 therapy in females. The literature describes reports of transient menstrual cycle irregularities, including amenorrhea, and ovarian failure in females treated with cumulative doses of 1,000 MBq to 59,000 MBq (27 mCi to 1,595 mCi) sodium iodide I-131. In a published literature analysis, the effects on fertility occurred in up to 30% of women treated with sodium iodide I-131, and may resolve 12 months after treatment.Males
Fertility may be impaired with Sodium Iodide I-131 Solution treatment. Discuss sperm banking for males who are expected to receive a high cumulative dose of sodium iodide I-131. Transient dose-related impairment of testicular function after sodium iodide I-131 therapy has been reported in the published literature. The literature describes reports of males treated with sodium iodide I-131 at doses of 370 MBq to 22,000 MBq (10 mCi to 595 mCi) resulting in transiently impaired testicular function (including spermatogenesis). The risk of persistent testicular dysfunction increases after administration of repeated or high cumulative radioiodide exposure.8.4 Pediatric Use
The safety and effectiveness of Sodium Iodide I-131 Solution have not been established in pediatric patients. Pediatric patients are at an increased lifetime risk for malignancy from radiation exposure.
8.5 Geriatric Use
Clinical experience has not identified differences in safety or effectiveness in geriatric patients compared to younger patients. However, elderly patients are more likely to have decreased renal function and radiation exposure is greater in patients with impaired renal function [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Sodium Iodide I 131 is primarily excreted by the kidneys. Renal function impairment decreases excretion of sodium iodide I 131 and increases the radiation exposure and risk of radiation toxicity. For patients with a history of renal impairment, evaluate renal function for therapeutic planning and consider dosimetry. Sodium Iodide I 131 is dialyzable. Hemodialysis can be used to reduce total body radiation exposure [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In case of exposure to a radioactive dose of sodium iodide I 131 exceeding the intended therapeutic dose, provide general supportive care, promote frequent voiding, monitor for bone marrow and thyroid suppression. Consider administering a thyroid blocking agent (e.g. potassium iodide (KI) or perchlorate) promptly within 4 to 6 hours after the exposure. Assess the benefit of administering a thyroid blocking agent against the risk of failure of sodium iodide I 131 therapy. Appropriate replacement therapy is recommended if hypothyroidism occurs.
-
11 DESCRIPTION
11.1 Chemical Characteristics
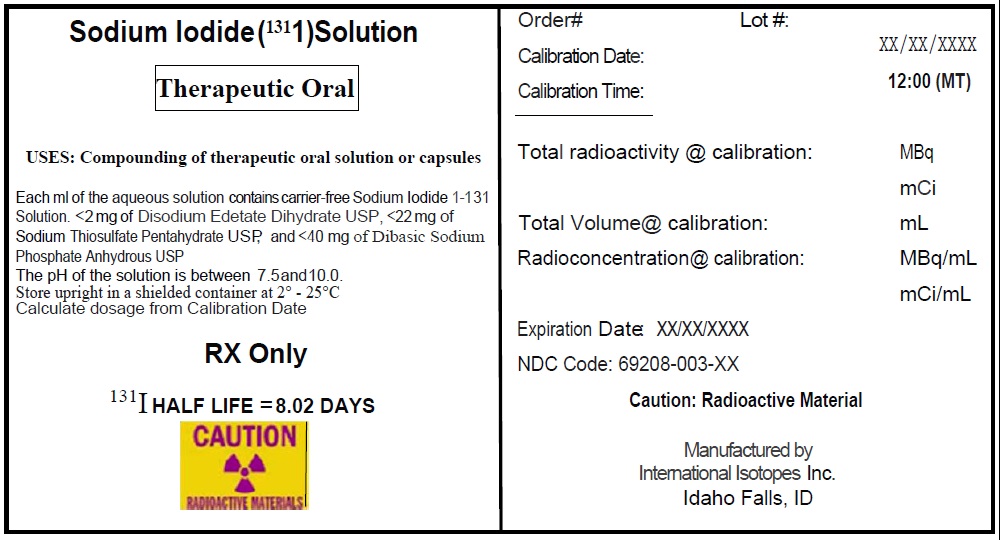
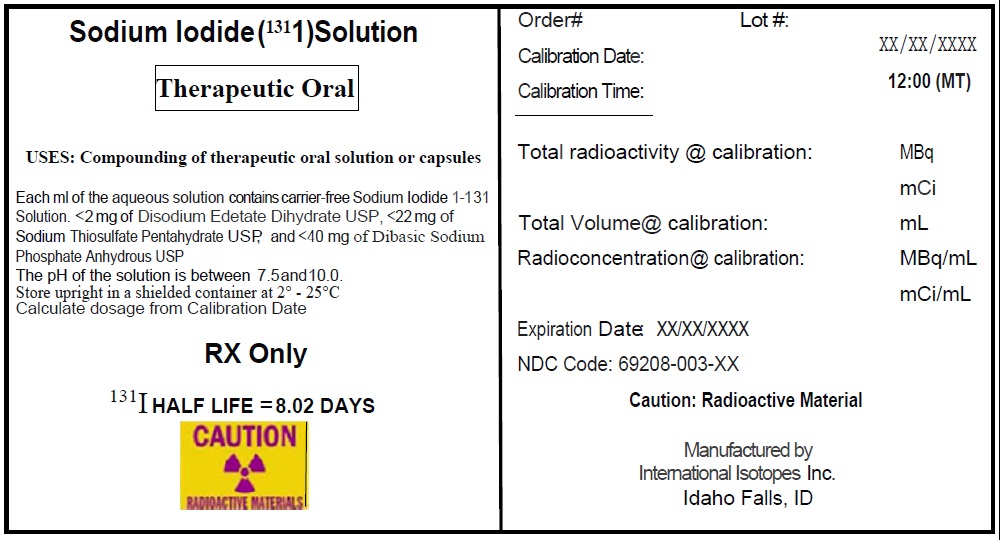
Sodium Iodide I-131 Solution, a radioactive therapeutic agent, provides a concentrated solution of sodium iodide I-131 with a radioconcentration of 37,000 MBq/mL (1,000 mCi/mL). Each mL of the concentrated solution contains 37,000 MBq of no-carrier-added sodium iodide I-131, disodium edetate dihydrate USP as a stabilizer, sodium thiosulfate pentahydrate USP as a reducing agent, and dibasic sodium phosphate anhydrous USP. The pH of the concentrated solution is between 7.5 and 10.
The concentrated solution provided with Sodium Iodide I-131 Solution is used for the preparation of sodium iodide I-131 capsules or sodium iodide I-131 solution of varying strengths for oral administration for therapy.
Sodium iodide I-131 solution is designated chemically as Na 131I and has a molecular weight of 153.99 g/mol. Hard gelatin capsules, provided for the preparation of the sodium iodide I-131 capsules final dosage form, contain approximately 300 mg of dibasic sodium phosphate anhydrous USP as the absorbing buffer.
11.2 Physical Characteristics
Iodine I-131 decays by beta emission and associated gamma emission with a physical half-life of 8.02 days. The principal radiation emissions are listed in Table 5.
Table 5 Principal Radiation Emission Data from Decay of Sodium Iodide I 131 Radiation Mean % per
DisintegrationMean Energy
(keV)Beta-1 2.1% 69.4 Beta-3 7.2% 96.6 Beta-4 89.4% 191.6 Gamma-7 6.1% 284.3 Gamma-14 81.2% 364.5 Gamma-18 7.1% 637.0 11.3 External Radiation
The specific gamma-ray constant for iodide I 131 is 4.26 × 10 -13 C·m 2·kg -1·MBq -1·s -1 (2.2 R·cm 2/mCi·hr). The first half-value thickness of lead (Pb) for iodide I 131 is 0.27 cm. A range of values for the relative attenuation of the radiation emitted by iodide I 131 that results from interposition of various thicknesses of Pb is shown in Table 6. For example, the use of 2.59 cm of Pb will decrease the external radiation exposure by a factor of about 1,000.
Table 6 Radiation Attenuation of Iodine I 131 by Lead Shielding Shield Thickness
(Pb) cmCoefficient of Attenuation 0.27 0.5 0.56 0.25 0.99 10 -1 2.59 10 -2 4.53 10 -3 To correct for physical decay of iodine I 131, the fractions that remain at selected intervals after the time of calibration are shown in Table 7.
Table 7 Physical Decay Chart: Iodine I-131 Half-Life 8.02 days *Calibration time Days Fraction Remaining Days Fraction Remaining Days Fraction Remaining 0* 1.000 11 .386 22 .149 1 .917 12 .354 23 .137 2 .841 13 .325 24 .126 3 .772 14 .298 25 .115 4 .708 15 .274 26 .106 5 .649 16 .251 27 .097 6 .595 17 .230 28 .089 7 .546 18 .211 29 .082 8 .501 19 .194 30 .075 9 .459 20 .178 10 .421 21 .163 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Iodide is actively transported by the sodium-iodide symporter (NIS) protein, in thyroid follicular cells. Iodide is concentrated in follicular cells to levels up to 50 times higher than in the plasma. Iodide is metabolically oxidized by thyroid peroxidase to iodinium (I +) which in turn iodinates tyrosine residues of thyroglobulin (tri or tetra-iodinated tyrosine). The beta emission of I 131 is responsible for the therapeutic effect.
12.2 Pharmacodynamics
The relationship between the extent of iodide I 131 exposure and pharmacologic effects has not been explored in clinical trials.
12.3 Pharmacokinetics
Absorption
Following oral administration of sodium iodide I 131, 90% of the administered radioactivity of Iodide I 131 is systemically absorbed in the first 60 minutes.Distribution
Following absorption, I 131 is distributed within the extra-cellular space. It is actively transported by the sodium-iodide symporter (NIS) protein, and binds to thyroglobulin resulting in accumulation in the thyroid. The thyroid uptake of iodide is usually increased in hyperthyroidism and in goiter, and is decreased in hypothyroidism. Sodium Iodide I 131 also accumulates in the stomach, choroid plexus, salivary glands, breast, liver, gall bladder, and kidneys.Elimination
Metabolism
In thyroidal follicular cells iodide is oxidized through the action of thyroid peroxidase to iodinium (I +) which in turn iodinates tyrosine residues of thyroglobulin.Excretion
Sodium iodide I 131 is excreted in urine and feces. The normal range of urinary excretion is 37% to 75% of the administered dose, varying with the thyroid and renal function of the patient. Fecal excretion is about 10%. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
The Sodium Iodide I-131 Solution provides a concentrated solution of sodium iodide I-131 with a radioconcentration of 37,000 MBq/mL (1,000 mCi/mL) at the time of calibration and can be supplied in any of the following vials: 1, 2 and 3 mL clear glass V-vials.
The concentrated solution is intended for use in the preparation of capsules and solutions of varying strengths for oral administration.
Concentrated Sodium Iodide I-131 Solution NDC Code Size/type Container 69208-001-15 1 mL V-vial 69208-001-25 2 mL V-vial 69208-001-35 3 mL V-vial A minimum of one carton of capsules containing 2 blister packs of capsules may be provided with each shipment. Each blister pack includes 5 empty #1 capsules and 5 filled #2 capsules containing approximately 300 mg of dibasic sodium phosphate anhydrous USP as the absorbing buffer. The capsules may be supplied along with the Sodium Iodide I-131 Solution for the preparation of sodium iodide I-131 capsules, therapeutic.
16.2 Storage
The Sodium Iodide I-131 Solution should be stored between 2 °C and 25 °C (36 °F and 77 °F). Store and dispose of Sodium Iodide I-131 Solution in compliance with the appropriate regulations of the government agency authorized to license the use of this radionuclide. Use Sodium Iodide I-131 Solution per the expiry date on the lead shield label. Use prepared capsules within 7 days of preparing.
Discard unused capsules after all Sodium Iodide I-131Solution has been dispensed or expired. New blister packages of hard gelatin capsules may be provided with each new shipment of Sodium Iodide I-131 Solution.
This radiopharmaceutical is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
-
17 PATIENT COUNSELING INFORMATION
Radiation Safety Precautions [see Warnings and Precautions (5.7)] .
- Advise patients treated for hyperthyroidism to monitor for and seek medical care for signs and symptoms of thyrotoxicosis and thyroid storm arising during the post-treatment period. For mild radiation-induced thyroiditis, patients may be advised to consider symptomatic management with pain-relievers or anti-inflammatory medications [see Warnings and Precautions (5.1)] .
- Advise patients to hydrate and void frequently and to use a sialagogue after administration of radioiodide to minimize radiation dose [see Warnings and Precautions (5.2)] .
- Advise patients to avoid close contact with others, especially pregnant women and children, and to take care to avoid contamination of other persons or the environment with body fluids [see Warnings and Precautions (5.7)] .
Embryo-Fetal Toxicity
- Advise female patients of the risk to a fetus [see Warnings and Precautions (5.4) , Use in Specific Populations (8.1)] .
- Advise females and males of reproductive potential to use effective contraception during treatment with sodium iodide I 131 and for at least 6 months after the last dose [see Warnings and Precautions (5.4) , Use in Specific Populations (8.3)] .
- Advise female patients to contact their healthcare provider with a known or suspected pregnancy.
Lactation
• Instruct women to stop breastfeeding and breast-pumping at least 6 weeks prior to sodium iodide I 131 administration [see Contraindications (4), Warnings and Precautions (5.5) and Use in Specific Populations (8.2)].Effects on Fertility
• Advise females and males of reproductive potential of the potential for impaired fertility with Sodium Iodide I-131 treatment [see Warnings and Precautions (5.6) and Use in Specific Populations (8.3)].Manufactured by:
Idaho Falls, Idaho 83401, USA
1-208-524-5300
www.intisoid.com
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SODIUM IODIDE I-131
sodium iodide i-131 kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69208-000 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69208-000-00 1 in 1 BOX 03/16/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, DISPENSING 1 Part 2 0 BLISTER PACK 1 Part 1 of 2 SODIUM IODIDE I-131
sodium iodide i-131 solution, concentrateProduct Information Item Code (Source) NDC:69208-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM IODIDE I-131 (UNII: 29VCO8ACHH) (IODIDE ION I-131 - UNII:4GC1FOQ22U) IODIDE ION I-131 1000 mCi Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM THIOSULFATE (UNII: HX1032V43M) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69208-003-15 1 in 1 VIAL, DISPENSING; Type 0: Not a Combination Product 2 NDC:69208-003-25 2 in 1 VIAL, DISPENSING; Type 0: Not a Combination Product 3 NDC:69208-003-35 3 in 1 VIAL, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209166 03/16/2020 Part 2 of 2 SODIUM PHOSPHATE
sodium phosphate capsules capsuleProduct Information Item Code (Source) NDC:69208-002 Route of Administration ORAL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) 300 mg Product Characteristics Color white (opaque) Score no score Shape CAPSULE Size 17mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BOX 1 2 in 1 CARTON 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209166 03/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209166 03/16/2020 Labeler - International Isotopes Inc (933155509) Registrant - International Isotopes Inc (933155509) Establishment Name Address ID/FEI Business Operations International Isotopes Inc 933155509 manufacture(69208-000, 69208-003, 69208-002) , api manufacture(69208-003, 69208-002)