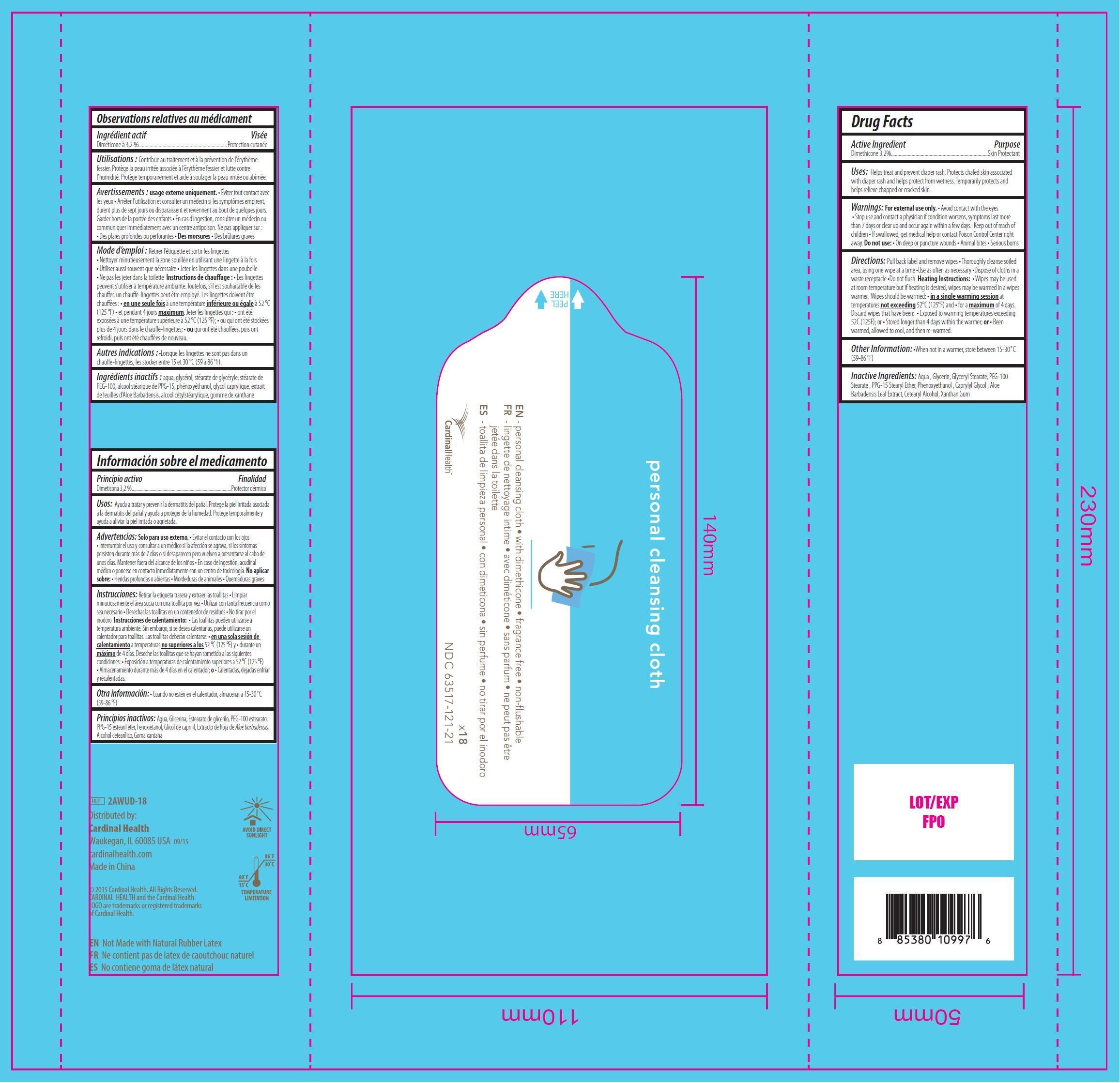
PERSONAL CLEANSING CLOTHS WITH DIMETHICONE- dimethicone swab
Cardinal Health 200, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Personal Cleansing Cloths with Dimethicone, 18 count
Uses:
Helps treat and prevent diaper rash. Protects chafed skin associated with diaper rash and helps protect from wetness. Temporarily protects and helps relieve chapped or cracked skin.
Warnings:
For external use only.
- Avoid contact with the eyes
Stop use
and contact a physician if condition worsens, symptoms last more than 7 days or clear up and occur again within a few days.
Directions:
Pull back label and remove wipes
• Thoroughly cleanse soiled area, using one wipe at a time
•Use as often as necessary
•Dispose of cloths in a waste receptacle
•Do not flush
Heating Instructions:
• Wipes may be used at room temperature but if heating is desired, wipes may be warmed in a wipes warmer. Wipes should be warmed:
• in a single warming session at temperatures not exceeding 52°C (125°F) and
• for a maximum of 4 days. Discard wipes that have been:
• Exposed to warming temperatures exceeding 52C (125F); or
• Stored longer than 4 days within the warmer; or
• Been warmed, allowed to cool, and then re-warmed.
| PERSONAL CLEANSING CLOTHS WITH DIMETHICONE
dimethicone swab |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Cardinal Health 200, Inc. (961027315) |