Label: GENTEAL TEARS MODERATE PRESERVATIVE FREE- dextran 70, hypromellose 2910 solution/ drops
- NDC Code(s): 0065-8063-01
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
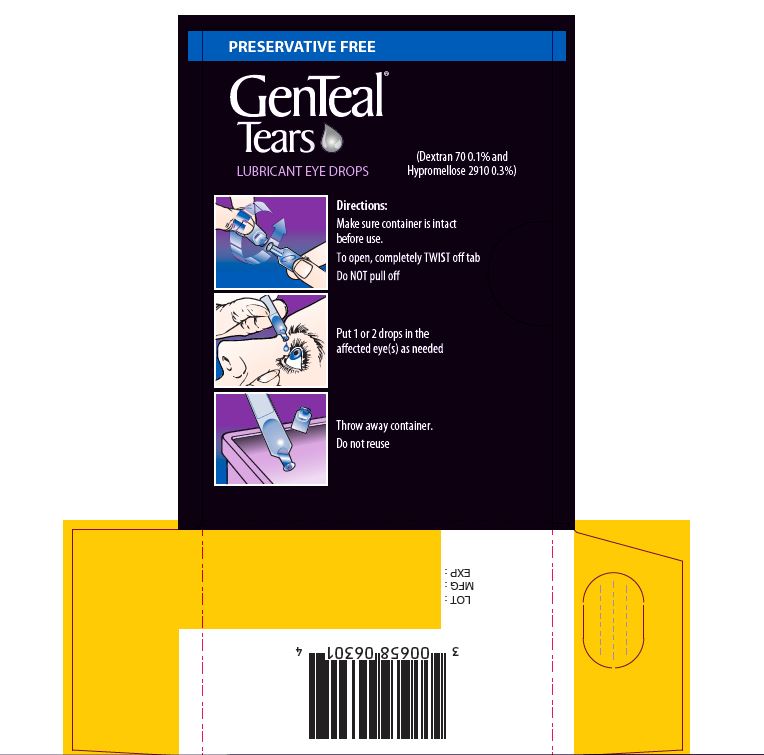
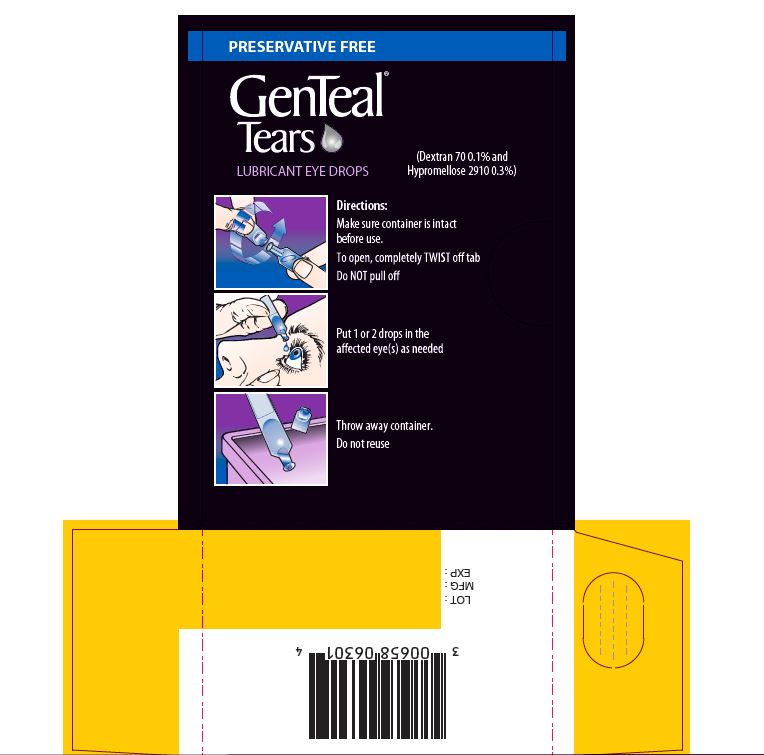
- Directions
- Other information
- Inactive ingredients
- Questions?
-
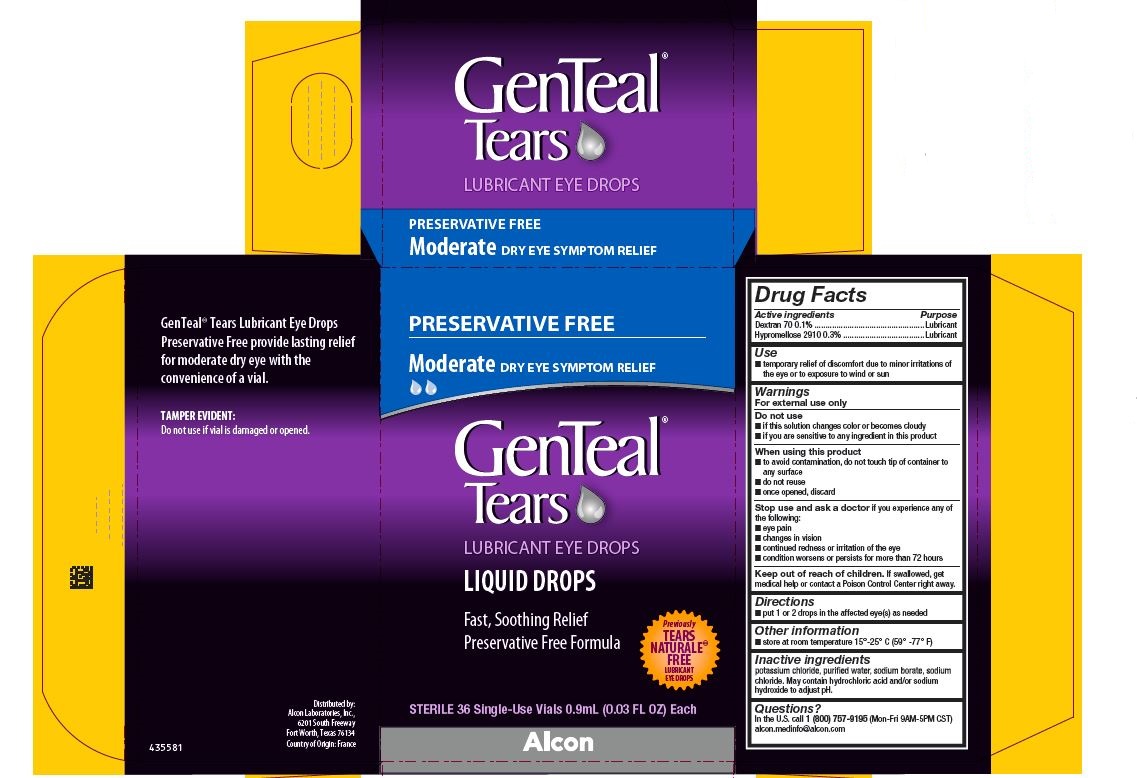
PRINCIPAL DISPLAY PANEL
PRESERVATIVE FREE
Moderate DRY EYE SYMPTOM RELIEF
GenTeal® Tears
LUBRICANT EYE DROPS
LIQUID DROPS
Fast, Soothing Relief
Preservative Free Formula
Previously TEARS NATURALE® FREE LUBRICANT EYE DROPS
STERILE 36 Single-Use Vials 0.9 mL (0.03 FL OZ) Each
Alcon
GenTeal® Tears Lubricant Eye Drops
Preservative Free provide lasting relief
for moderate dry eye with the
convenience of a vial.
TAMPER EVIDENTY:
Do not use if vial is damaged or opened.
Distributed by:
Alcon Laboratories, Inc.,
6201 South Freeway
Fort Worth, Texas 76134
Country of Origin: France
435581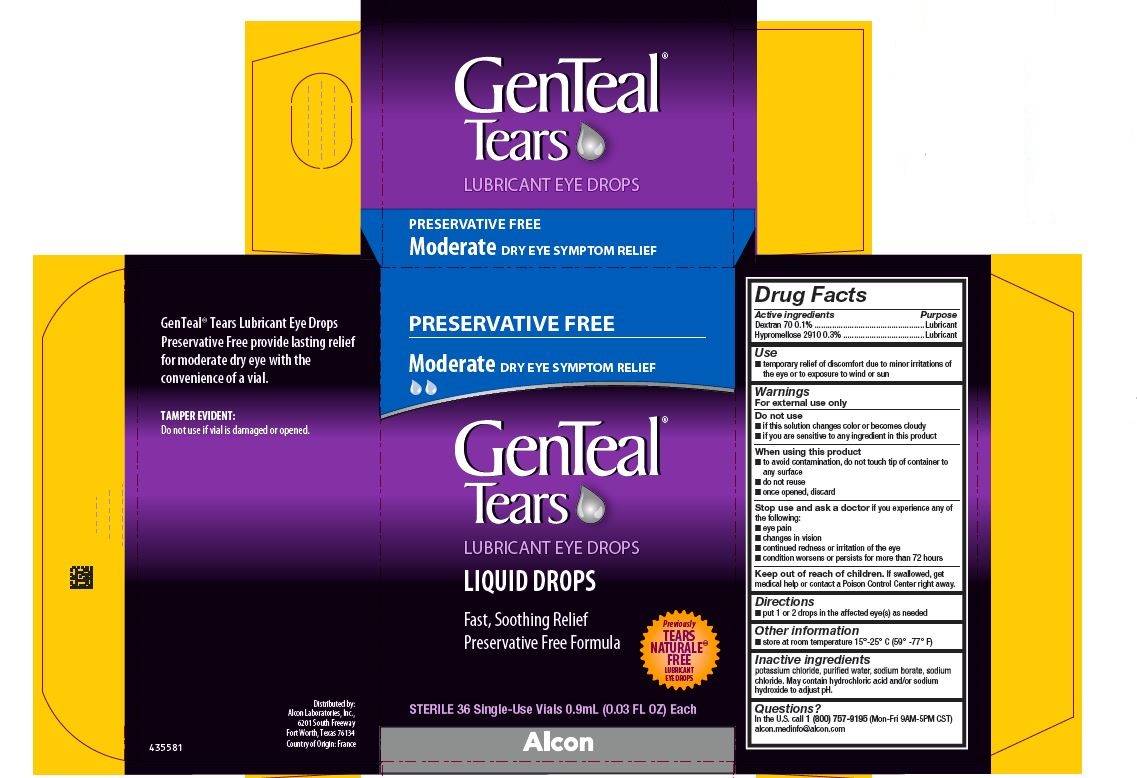
-
INGREDIENTS AND APPEARANCE
GENTEAL TEARS MODERATE PRESERVATIVE FREE
dextran 70, hypromellose 2910 solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-8063 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hypromellose 2910 (4000 Mpa.s) (UNII: RN3152OP35) (Hypromellose 2910 (4000 Mpa.s) - UNII:RN3152OP35) Hypromellose 2910 (4000 Mpa.s) 3 mg in 1 mL Dextran 70 (UNII: 7SA290YK68) (Dextran 70 - UNII:7SA290YK68) Dextran 70 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Potassium Chloride (UNII: 660YQ98I10) Water (UNII: 059QF0KO0R) Sodium Borate (UNII: 91MBZ8H3QO) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Sodium Chloride (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-8063-01 36 in 1 CARTON 07/03/2018 1 .9 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 07/03/2018 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Kaysersberg Pharmaceuticals 267486052 manufacture(0065-8063)