Label: ORAJEL COLD SORE MOISTURELOCK- benzocaine cream
- NDC Code(s): 10237-761-01
- Packager: Church & Dwight Co., Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 13, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
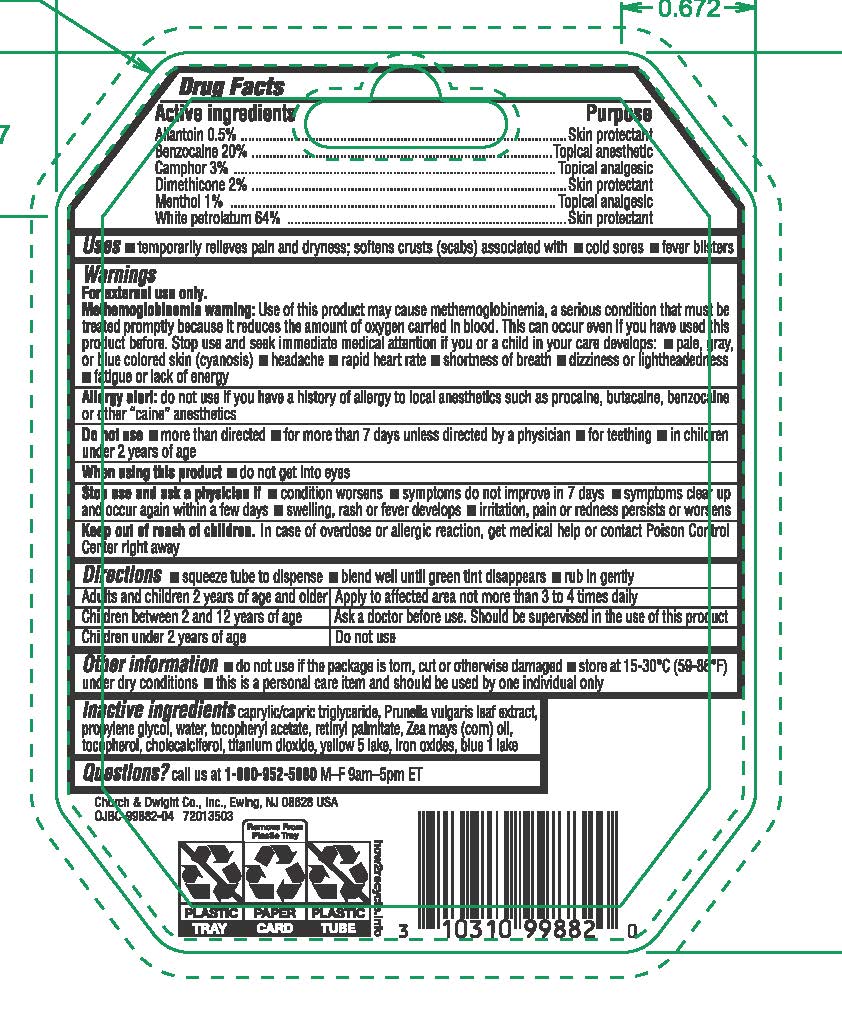
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
• squeeze tube to dispense • blend well until green tint disappears • rub in gently
Adults and children 2 years of age and older | Apply to affected area not more than 3 to 4 times daily
Children under 12 years of age | Should be supervised in the use of this product
Children under 2 years of age | Ask a physician
Other information • do not use if the package is torn, cut or otherwise damaged • store at 15-30ºC (59-86º) under dry conditions • this is a personal care item and should be used by one individual only
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORAJEL COLD SORE MOISTURELOCK
benzocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10237-761 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 5 mg in 1 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 30 mg in 1 g DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 640 mg in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PRUNELLA VULGARIS LEAF (UNII: 2LW0610U4O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CORN OIL (UNII: 8470G57WFM) CHOLECALCIFEROL (UNII: 1C6V77QF41) FERRIC OXIDE RED (UNII: 1K09F3G675) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) TOCOPHEROL (UNII: R0ZB2556P8) Product Characteristics Color green Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10237-761-01 1 in 1 CARTON 07/01/2016 1 3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 07/01/2016 Labeler - Church & Dwight Co., Inc. (001211952) Establishment Name Address ID/FEI Business Operations Accupac 071609663 manufacture(10237-761)