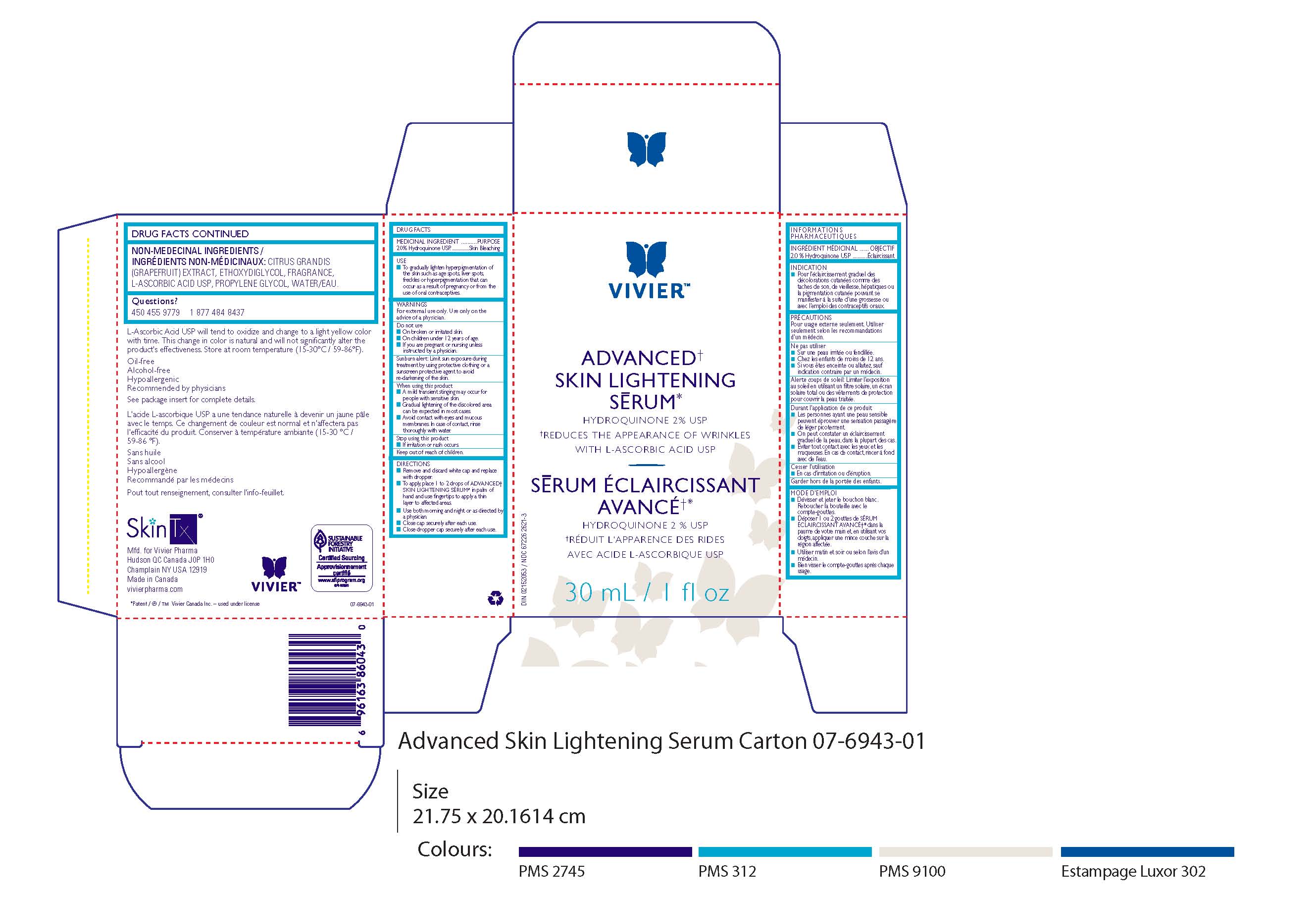
ADVANCED SKIN LIGHTENING SERUM- hydroquinone liquid
Vivier Pharma, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Advanced Skin Lightening Serum
Active Ingredient
Hydroquinone 2% USP in a nonmedicinal liquid gel base containing Vitamin C IDS™ (L-Ascorbic Acid USP).
Indication
To gradually lighten hyperpigmentation of the skin such as age spots, liver spots, freckles, or other areas of unwanted melanin hyperpigmentation that may occur as a result of pregnancy or from the use of oral contraceptives.
Precautions
For external use only. Avoid contact with eyes and mucous membranes. If contact occurs, rinse thoroughly with water. Keep out of
reach of children. Vitamin C (L-Ascorbic Acid USP) may cause a slight tingling sensation in some people. This is normal and will usually diminish with time. See package insert for more details.
Directions
Remove and discard white cap and replace with dropper. Place two to three drops in palm of hand and use fingertips to apply a thin layer
to face, neck, chest and back of hands as desired. Use both morning and night or as directed by a physician. Close dropper cap securely after each use. Gradual lightening of the discoloration can be expected in most cases. L-Ascorbic Acid USP will tend to oxidize and change to a light yellow color with time. This change in color is natural and will not significantly alter the product’s effectiveness. Store at room temperature (15-25ºC / 59-77ºF).
| ADVANCED SKIN LIGHTENING SERUM
hydroquinone liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Vivier Pharma, Inc. (250996550) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Entreprises ImportFAB Inc. | 248586117 | manufacture(67226-2621) | |